Aortic Coarctation and Interrupeted
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Patent Ductus Arteriosus
Peer Reviewed Correction of a Canine Left-to-Right Shunting By Connie K. Varnhagen, PhD, RAHT Patent Ductus Arteriosus Scrappy, a 5-year-old, 27-kg, neutered Labrador retriever, presented to the Calgary Animal Referral and Emergency (CARE) Centre in Calgary, Alberta, Canada, for mild exercise intolerance. The owner reported that the normally active dog was “slowing down” and pant- ing excessively even after mild exercise. History time and mucous membrane color were normal. Lung sounds The patient was the runt of its litter, was rejected by its were normal on auscultation. Based on the physical examina- dam, and was hand-fed for the first 5 days. A presumed tion, the differential diagnosis included patent ductus arterio- innocent heart murmur was detected on auscultation at the sus (PDA) or another type of arteriovenous fistula. puppy’s first veterinary examination. Right lateral and ventrodorsal (Figure 1) thoracic radio- At approximately 6 months of age, the patient began graphs revealed cardiomegaly with left atrial and ventricu- experiencing chronic small intestine diarrhea and weight lar enlargement and a prominent pulmonary artery bulge. loss. Over the next 30 months, the dog was diagnosed and Electrocardiography (ECG; Figure 2) demonstrated a tall treated for giardiasis, coccidiosis, small intestinal bacterial R wave (greater than 4.0 mV in lead II compared with a overgrowth, borderline exocrine pancreatic insufficiency, normal parameter of 3.0 mV), indicative of left ventricular and lymphocytic plasmacytic inflammatory bowel disease. enlargement, and normal sinus rhythm. Also at approximately 6 months of age, the patient Echocardiography was performed and was definitive developed a nonseasonal pruritus and dry, brittle haircoat. -
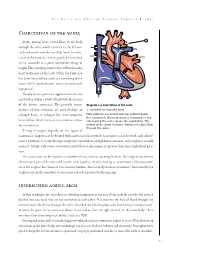
Coarctation of the Aorta Interrupted Aortic Arch
T HE P EDIATRIC C ARDIAC S URGERY I NQUEST R EPORT Coarctation of the aorta In the normal heart, blood flows to the body through the aorta, which connects to the left ven- tricle and arches over the top of the heart.In coarc- tation of the aorta,the aorta is pinched (in medical terms, coarcted) at a point somewhere along its length. This pinching restricts blood flow from the heart to the rest of the body. Often, the baby also has other heart defects, such as a narrowing of the aortic arch (in medical terms,transverse aortic arch hypoplasia). Usually no symptoms are apparent at birth, but can develop within a week of birth with the closure of the ductus arteriosus. The possible conse- Diagram 2.9 Coarctation of the aorta quences of this condition are poor feeding, an 1 – pinched or coarcted aorta enlarged heart, an enlarged liver and congestive Flow patterns are normal but are reduced below the coarctation. Blood pressure is increased in ves- heart failure. Blood pressure also increases above sels leaving the aorta above the coarctation. The the constriction. broken white arrow indicates diminished blood flow through the aorta. Timing of surgery depends on the degree of coarctation. Surgery may be delayed with mild coarctation (which sometimes is not detected until adoles- cence). However, if a baby develops congestive heart failure or high blood pressure, early surgery is usually required. A baby with severe coarctation should have early surgery to prevent long-term high blood pres- sure. The coarctation can be repaired in a number of ways without opening the heart.The surgeon can remove the narrowed part of the aorta and sew the ends together, thereby creating an anastomosis. -

Patent Ductus Arteriosus About This Factsheet the Normal Heart
Understanding your child’s heart Patent ductus arteriosus About this factsheet The normal heart This factsheet is for parents of babies and children who The heart is a muscular pump which pumps blood through the have patent ductus arteriosus (PDA), which is also known as body and lungs. There are four chambers in the heart. The two persistent arterial duct. upper ones are called the right atrium and left atrium. These are separated by a wall called the atrial septum. The two lower It explains: chambers are called the right and left ventricles, and are separated • what patent ductus arteriosus is and how it is diagnosed by a wall called the ventricular septum. • how patent ductus arteriosus is treated • the benefits and risks of treatments. On each side of the heart, blood passes from the atrium, through a heart valve – the tricuspid valve on the right, and the mitral valve This factsheet does not replace the advice that doctors or on the left – into the ventricle. The ventricles are the main pumping nurses may give you, but it should help you to understand chambers of the heart. Each ventricle pumps blood out into an artery. what they tell you. The right ventricle pumps blood – blue in the illustration – into the pulmonary artery (the blood vessel that takes blood to the lungs). The left ventricle pumps blood – red in the illustration – into the aorta (the blood vessel that takes blood to the rest of the body). Blood flows from the right side of the heart, through the pulmonary valve into the pulmonary artery, and then to the lungs where it picks up oxygen. -

Echocardiography of the Patent Ductus Arteriosus in Premature Infant
Received: 15 August 2018 | Accepted: 16 October 2018 DOI: 10.1111/chd.12703 SPECIAL ISSUE ARTICLE Echocardiography of the patent ductus arteriosus in premature infant Govinda Paudel MD | Vijaya Joshi MD Pediatric Cardiology, University of Tennessee Health Science Center, Le Abstract Bonheur Children’s Hospital, Memphis, Management of the patent ductus arteriosus (PDA) in the premature infant has been Tennessee a point of controversy for decades as smaller and earlier gestational age infants have Correspondence been surviving. Increasing experience with catheter‐based device closure has gener‐ Govinda Paudel, MD, Pediatric Cardiology, University of Tennessee Health Science ated a new wave of interest in this subject. In this era, echocardiography plays a cen‐ Center, Le Bonheur Children’s Hospital, 49 tral role for collaboration within a multispecialty team. Reliability of echocardiography North Dunlap Street, Memphis, TN, 38103. Email: [email protected] is improved by applying an institutionally derived standard approach to imaging, data collection, and reporting. The key aspects of both the physiology and anatomy of the PDA to distinguish infants that may benefit from intervention are described. KEYWORDS device closure, echocardiography, patent ductus arteriosus, premature infants 1 | INTRODUCTION cardiologists (including interventionist) and neonatologists should agree on the imaging parameters desired and reported. The report Patent ductus arteriosus (PDA) is associated with numerous com‐ summary should have a standard format that tracks serial changes plications in premature babies.1 Hemodynamics of the PDA guide from the previous echo as this is essential to optimize communication. management. But anatomic features have gained importance with Although high‐frequency probes (8‐12 MHz) are most commonly the growing experience in transcatheter device closure of the ductus utilized, lower frequency probes can provide better penetration from in premature infants. -

The Patent Ductus Arteriosus (PDA) and the Preterm Baby
12/5/2018 The Patent Ductus Arteriosus (PDA) and the Preterm Baby Tanya Hatfield, RNC - NIC, MSN Neonatal Outreach Educator Objectives ▪ Describe normal cardiac physiology and development ▪ Understand the unique physiologic needs of the preterm infant with a PDA ▪ Define the implications prematurity presents with the cardiac system 2 1 12/5/2018 Normal Cardiovascular Function: Review Uptodate.com,. (2015). Identifying newborns with critical congenital heart disease. Retrieved 22 October 2015, 3 from http://www.uptodate.com/contents/identifying - newborns - with - critical - congenital - heart - disease?source=search_result&search=congenital+heart+disease&selectedTitle=1~150 Normal Cardiovascular Function: Review Right Left Lungs Body Pulmonary Systemic Artery (PA) Aorta ( Ao ) Uptodate.com,. (2015). Identifying newborns with critical congenital heart disease. Retrieved 22 October 2015, 4 from http://www.uptodate.com/contents/identifying - newborns - with - critical - congenital - heart - disease?source=search_result&search=congenital+heart+disease&selectedTitle=1~150 2 12/5/2018 Fetal Circulation (2015). Retrieved 29 October 2015, from 5 http://higheredbcs.wiley.com/legacy/college/tortora/0470565101/hearthis_ill/pap13e_ch21_illustr_audio_m p3_am/simulations/hear/fetal_circulation.html Fetal Circulation ▪ Gas exchange is liquid to liquid ▪ Organ of respiration is placenta ▪ High flow, low resistance ▪ Fetal lungs • Low flow, high resistance ▪ PA’s constricted ▪ High right heart and lung pressures ▪ Low left heart pressures ▪ Open fetal -

Artery Umbilical Cord
THE PRACTICAL IMPORTANCE OF THE SINGLE ARTERY UMBILICAL CORD RICHARD F. HNAT Department of Obstetrics and Gynecology, The New York Hospital-Cornell Medical Center, New Yoi [Received 23rd May 1966) Summary. The correlation of single artery umbilical cords with con- genital anomalies is explored in the present study of 4808 consecutive deliveries. There were thirty-eight single artery cords, and six of these were associated with additional foetal abnormalities. An attempt is made to present the correlation in a true perspective by adding these data to other similar studies. Acknowledgment is given to the many retrospective studies, but consecutive delivery series show that a single artery cord is of importance as an abnormal physical finding but not as a screening test to detect occult malformations. INTRODUCTION Recent studies from large obstetrical services have suggested a correlation between the absence of one umbilical artery and congenital anomalies. Although this association had been mentioned before and after the observa¬ tion attributed to Hyrtl, it attracted little attention until the work of Ben- irschke & Brown (1955), a retrospective study of fifty-five placentae and autopsies of newborns from three different institutions. Of these infants, twenty-seven exhibited malformations involving all organ systems. Since 1955 many reports have appeared. Retrospective studies have taken the form of either autopsy material or accumulated cases, usually from different hospitals, added to the experience of the large teaching institution from which the report emanated (Table 1). Prospective studies consist entirely of cord specimens collected from consecutive deliveries, and they have consequently suffered from the small number of cases (Table 2). -

Thoracic Aorta
GUIDELINES AND STANDARDS Multimodality Imaging of Diseases of the Thoracic Aorta in Adults: From the American Society of Echocardiography and the European Association of Cardiovascular Imaging Endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance Steven A. Goldstein, MD, Co-Chair, Arturo Evangelista, MD, FESC, Co-Chair, Suhny Abbara, MD, Andrew Arai, MD, Federico M. Asch, MD, FASE, Luigi P. Badano, MD, PhD, FESC, Michael A. Bolen, MD, Heidi M. Connolly, MD, Hug Cuellar-Calabria, MD, Martin Czerny, MD, Richard B. Devereux, MD, Raimund A. Erbel, MD, FASE, FESC, Rossella Fattori, MD, Eric M. Isselbacher, MD, Joseph M. Lindsay, MD, Marti McCulloch, MBA, RDCS, FASE, Hector I. Michelena, MD, FASE, Christoph A. Nienaber, MD, FESC, Jae K. Oh, MD, FASE, Mauro Pepi, MD, FESC, Allen J. Taylor, MD, Jonathan W. Weinsaft, MD, Jose Luis Zamorano, MD, FESC, FASE, Contributing Editors: Harry Dietz, MD, Kim Eagle, MD, John Elefteriades, MD, Guillaume Jondeau, MD, PhD, FESC, Herve Rousseau, MD, PhD, and Marc Schepens, MD, Washington, District of Columbia; Barcelona and Madrid, Spain; Dallas and Houston, Texas; Bethesda and Baltimore, Maryland; Padua, Pesaro, and Milan, Italy; Cleveland, Ohio; Rochester, Minnesota; Zurich, Switzerland; New York, New York; Essen and Rostock, Germany; Boston, Massachusetts; Ann Arbor, Michigan; New Haven, Connecticut; Paris and Toulouse, France; and Brugge, Belgium (J Am Soc Echocardiogr 2015;28:119-82.) TABLE OF CONTENTS Preamble 121 B. How to Measure the Aorta 124 I. Anatomy and Physiology of the Aorta 121 1. Interface, Definitions, and Timing of Aortic Measure- A. The Normal Aorta and Reference Values 121 ments 124 1. -

Patent Ductus Arteriosus with Pulmonary Hypertension by John A
Br Heart J: first published as 10.1136/hrt.15.4.423 on 1 October 1953. Downloaded from PATENT DUCTUS ARTERIOSUS WITH PULMONARY HYPERTENSION BY JOHN A. COSH From the Departnent ofMedicine, University of Bristol Received July 22, 1953 In most cases of patent ductus arteriosus the diagnosis is readily made on recognition of the typical murmur. In some, however, the continuous murmur is lacking, and a systolic murmur only, of variable loudness, is heard at the pulmonary area. This is not uncommon in infants and young children, who may later develop the characteristic murmur (Gilchrist, 1945). Occasionally the continuous murmur is absent in adults in whom patency of the ductus is found subsequently at autopsy: in such cases the state of the pulmonary arteries and the presence of right ventricular hypertrophy may indicate that the pulmonary arterial pressure was much raised in life (Holman, 1925; Keys and Shapiro, 1943 (Case 3); Chapman, 1944; Douglas et al., 1947; Ulrich, 1947). As a result, the pressure gradient between the aorta and the pulmonary artery may be so diminished that the blood flow along the ductus is insufficient to set up a continuous murmur. In extreme examples the pressure in the pulmonary artery may exceed that in the aorta, causing a reversal of the usual left to right shunt (Johnson et al., 1950; Campbell and Hudson, 1951; Dammann et al., 1953). Three cases of patent ductus arteriosus complicated by severe pulmonary hypertension are presented here. The diagnosis was made in each on cardiac catheterization. In two, pulmonary hypertension caused reversal of the shunt; in the third the shunt was not reversed, but there was, in addition, coarctation of the aorta. -

Fetal Circulation
The Fetal Circulation Dr. S. Mathieu, Specialist Registrar in Anaesthesia Dr. D. J. Dalgleish, Consultant Anaesthetist Royal Bournemouth and Christchurch Hospitals Trust, UK Questions 1. In the fetal circulation: a) There are two umbilical arteries and one umbilical vein? b) Over 90% of blood passes the liver via the ductus venosus c) The foramen ovale divides the left and right ventricle d) The umbilical artery carries oxygenated blood from the placenta to the fetus e) The foramen ovale allows oxygenated blood to bypass the pulmonary circulation 2. In the fetal circulation: a) The oxygen dissociation curve of fetal haemoglobin is shifted to the left compared with adult haemoglobin ensuring oxygen delivery to the fetus despite low oxygen partial pressures b) It is the presence of the ductus arteriosus and large pulmonary vascular resistance which ensures most of the right ventricular output passes into the aorta c) The patency of the ductus arteriosus is maintained by high oxygen tensions d) The patency of the ductus arteriosus is maintained by the vasodilating effects of prostaglandin G2 e) 2,3-DPG levels are higher in fetal haemoglobin compared with adult haemaglobin 3. Changes at birth include: a) a fall in pulmonary vascular resistance b) a rise in systemic vascular resistance with clamping of the cord c) an increase in hypoxic pulmonary vasoconstriction d) a rise in left atrial pressure e) closure of the ductus arteriosus within 24 hours 4. The following congenital heart lesions are cyanotic: a) Ventricular septal defect b) Atrial septal defect c) Patent ductus arteriosus d) Tetralogy of Fallot e) Transposition of the great arteries MCQ answers at end Key points • The fetal circulation supplies the fetal tissues with oxygen and nutrients from the placenta. -

Concomitant Stenting of the Patent Ductus Arteriosus And
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Alwi et al Congenital Heart Disease Concomitant stenting of the patent ductus arteriosus and radiofrequency valvotomy in pulmonary atresia with intact ventricular septum and intermediate right ventricle: Early in-hospital and medium-term outcomes Mazeni Alwi, MRCP,a Kok-Kuan Choo, MRCP,a Nomee A. M. Radzi, MRCPCH,a Hasri Samion, MD,a Kiew-Kong Pau, FRCS,b and Chee-Chin Hew, FRCSb Objectives: Our objective was to determine the feasibility and early to medium-term outcome of stenting the CHD patent ductus arteriosus at the time of radiofrequency valvotomy in the subgroup of patients with pulmonary atresia with intact ventricular septum and intermediate right ventricle. Background: Stenting of the patent ductus arteriosus and radiofrequency valvotomy have been proposed as the initial intervention for patients with intermediate right ventricle inasmuch as the sustainability for biventricular circulation or 1½-ventricle repair is unclear in the early period. Methods: Between January 2001 and April 2009, of 143 patients with pulmonary atresia and intact ventricular septum, 37 who had bipartite right ventricle underwent radiofrequency valvotomy and stenting of the patent duc- tus arteriosus as the initial procedure. The mean tricuspid valve z-score wasÀ3.8 Æ 2.2 and the mean tricuspid valve/mitral valve ratio was 0.62 Æ 0.16. Results: Median age was 10 days (3–65 days) and median weight 3.1 kg (2.4–4.9 kg). There was no procedural mortality. Acute stent thrombosis developed in 1 patient and necessitated emergency systemic–pulmonary shunt. -

Pulmonary Atresia: Intact Ventricular Septum
© 2012 The Children’s Heart Clinic Normal Heart NOTES: Children’s Heart Clinic, P.A., 2530 Chicago Avenue S, Ste 500, Minneapolis, MN 55404 West Metro: 612-813-8800 * East Metro: 651-220-8800 * Toll Free: 1-800-938-0301 * Fax: 612-813-8825 Children’s Minnesota, 2525 Chicago Avenue S, Minneapolis, MN 55404 West Metro: 612-813-6000 * East Metro: 651-220-6000 © 2012 The Children’s Heart Clinic Reviewed March 2019 Pulmonary Atresia with Intact Ventricular Septum (PA/IVS) Pulmonary atresia with intact ventricular septum (PA/IVS) refers to the absence or underdevelopment of the pulmonary valve and the absence of a communication between the lower two chamber of the heart (ventricles). The pulmonary valve ring and main pulmonary artery are hypoplastic (underdeveloped) due to lack of blood flow in utero. This means there is no direct communication between the right ventricle and the pulmonary artery. The only source of blood to the lungs is supplied by a patent ductus arteriosus (PDA), a normal fetal structure that usually closes in the first week of life. PA/IVS is often associated with coronary anomalies, such as obstruction or absence of the proximal or left coronary artery. The right ventricle (RV) size varies and portions of the right ventricle may be absent. The muscle (myocardium) of the right ventricle (RV) is often abnormal. PA/IVS accounts for less than 1% of all congenital heart defects and 2.5% of all critically ill infants with congenital heart disease. Physical Exam/Symptoms: Severe cyanosis (blue color) persists from birth. Tachypnea is often present. -

Pulmonary-Atresia-Mapcas-Pavsdmapcas.Pdf
Normal Heart © 2012 The Children’s Heart Clinic NOTES: Children’s Heart Clinic, P.A., 2530 Chicago Avenue S, Ste 500, Minneapolis, MN 55404 West Metro: 612-813-8800 * East Metro: 651-220-8800 * Toll Free: 1-800-938-0301 * Fax: 612-813-8825 Children’s Minnesota, 2525 Chicago Avenue S, Minneapolis, MN 55404 West Metro: 612-813-6000 * East Metro: 651-220-6000 © 2012 The Children’s Heart Clinic Reviewed March 2019 Pulmonary Atresia, Ventricular Septal Defect and Major Aortopulmonary Collateral Arteries (PA/VSD/MAPCAs) Pulmonary atresia (PA), ventricular septal defect (VSD) and major aortopulmonary collateral arteries (MAPCAs) is a rare type of congenital heart defect, also referred to as Tetralogy of Fallot with PA/MAPCAs. Tetralogy of Fallot (TOF) is the most common cyanotic heart defect and occurs in 5-10% of all children with congenital heart disease. The classic description of TOF includes four cardiac abnormalities: overriding aorta, right ventricular hypertrophy (RVH), large perimembranous ventricular septal defect (VSD), and right ventricular outflow tract obstruction (RVOTO). About 20% of patients with TOF have PA at the infundibular or valvar level, which results in severe right ventricular outflow tract obstruction. PA means that the pulmonary valve is closed and not developed. When PA occurs, blood can not flow through the pulmonary arteries to the lungs. Instead, the child is dependent on a patent ductus arteriosus (PDA) or multiple systemic collateral vessels (MAPCAs) to deliver blood to the lungs for oxygenation. These MAPCAs usually arise from the de- scending aorta and subclavian arteries. Commonly, the pulmonary arteries are abnormal, with hypoplastic (small and underdeveloped) central and branch pulmonary arteries and/ or non-confluent central pulmonary arteries.