Genetic Structure Characterization of Chileans Reflects Historical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

By Amalia Damgaard
By Private Chef Amalia Damgaard CHILEAN PANORAMA Although it appears slim and small, Chile is a long and narrow country about the size of Texas, with a vast coast line covering about 3,998 miles. The Pacific Ocean borders to the west; Argentina is a neighbor to the east; Bolivia, to the northeast; and Peru, to the north. Because of its geographical location, Chile has an unusual and fun landscape, with deserts, beaches, fjords, glaciers and icebergs, fertile lands, the Andes mountains, over 600 volcanoes (some active), and sub-artic conditions in the South. Since Chile is below the equator, their seasons are different from ours in the United States. So, when we have winter they have summer, and so on. Even though Chile had years of political and economic turmoil, it has evolved into a market-oriented economy with strong foreign trade. Currently, it has the strongest economy in South America, with a relatively-low crime rate, and a high standard of living. Chile is a land rich in beauty, culture, and literature. It is called “the Switzerland of South America” because of its natural splendor. World renowned poets, Pablo Neruda and Gabriela Mistral, won Nobel Prizes. The majority of Chileans are descendants of Europeans, namely Spanish, French, and German, and others in smaller numbers. Allegedly, the original inhabitants of the region prior to Spanish conquest were not natives but merely nomads who lived in the area. Their descendants are today about 3% of the population. A mixture of the so-called natives and European settlers is called “mestizo.” Today’s mestizos are so well blended that they look mostly European. -

Permanent War on Peru's Periphery: Frontier Identity
id2653500 pdfMachine by Broadgun Software - a great PDF writer! - a great PDF creator! - http://www.pdfmachine.com http://www.broadgun.com ’S PERIPHERY: FRONT PERMANENT WAR ON PERU IER IDENTITY AND THE POLITICS OF CONFLICT IN 17TH CENTURY CHILE. By Eugene Clark Berger Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in History August, 2006 Nashville, Tennessee Approved: Date: Jane Landers August, 2006 Marshall Eakin August, 2006 Daniel Usner August, 2006 íos Eddie Wright-R August, 2006 áuregui Carlos J August, 2006 id2725625 pdfMachine by Broadgun Software - a great PDF writer! - a great PDF creator! - http://www.pdfmachine.com http://www.broadgun.com HISTORY ’ PERMANENT WAR ON PERU S PERIPHERY: FRONTIER IDENTITY AND THE POLITICS OF CONFLICT IN 17TH-CENTURY CHILE EUGENE CLARK BERGER Dissertation under the direction of Professor Jane Landers This dissertation argues that rather than making a concerted effort to stabilize the Spanish-indigenous frontier in the south of the colony, colonists and indigenous residents of 17th century Chile purposefully perpetuated the conflict to benefit personally from the spoils of war and use to their advantage the resources sent by viceregal authorities to fight it. Using original documents I gathered in research trips to Chile and Spain, I am able to reconstruct the debates that went on both sides of the Atlantic over funds, protection from ’ th pirates, and indigenous slavery that so defined Chile s formative 17 century. While my conclusions are unique, frontier residents from Paraguay to northern New Spain were also dealing with volatile indigenous alliances, threats from European enemies, and questions about how their tiny settlements could get and keep the attention of the crown. -

THE PEOPLES and LANGUAGES of CHILE by DONALDD
New Mexico Anthropologist Volume 5 | Issue 3 Article 2 9-1-1941 The eoplesP and Languages of Chile Donald Brand Follow this and additional works at: https://digitalrepository.unm.edu/nm_anthropologist Recommended Citation Brand, Donald. "The eP oples and Languages of Chile." New Mexico Anthropologist 5, 3 (1941): 72-93. https://digitalrepository.unm.edu/nm_anthropologist/vol5/iss3/2 This Article is brought to you for free and open access by the Anthropology at UNM Digital Repository. It has been accepted for inclusion in New Mexico Anthropologist by an authorized editor of UNM Digital Repository. For more information, please contact [email protected]. 72 NEW MEXICO ANTHROPOLOGIST THE PEOPLES AND LANGUAGES OF CHILE By DONALDD. BRAND This article initiates a series in which the writer will attempt to summarize the scattered and commonly contradictory material on the present ethnic and linguistic constituency of a number of Latin Ameri- can countries. It represents some personal investigations in the field and an examination of much of the pertinent literature. Chile has been a sovereign state since the War of Independence 1810-26. This state was founded upon a nuclear area west of the Andean crest and essentially between 240 and 460 South Latitude. Through the War of the Pacific with Bolivia and Perui in 1879-1883 and peaceful agreements with Argentina, Chile acquired her present extention from Arica to Tierra del Fuego. These northern and south- ern acquisitions added little to her population but introduced numerous small ethnic and linguistic groups. Chile has taken national censuses in 1835, 1843, 1854, 1865, 1875, 1885, 1895, 1907, 1920, 1930, and the most recent one in November of 1940. -

Aspects of Social Justice Ally Work in Chilean Historical Fiction: the Case of the Pacification of Araucanía
Aspects of Social Justice Ally Work in Chilean Historical Fiction: The Case of the Pacification of Araucanía Katherine Karr-Cornejo Whitworth University Abstract Majority-culture writers often depict cultures different from their own, but approaching cultures to which an author does not belong can be challenging. How might we read dominant-culture portrayals of marginalized cultures that tell stories of injustice? In this paper I utilize the frame of identity development in social justice allies in order to understand the narratives dominant-culture authors use in fiction to reflect sympathetic views of indigenous justice claims. In order to do so, I study three historical novels set in Araucanía during the second half of the nineteenth century, considering historiographical orientation, representation of cultural difference, and understanding of sovereignty:Casas en el agua (1997) by Guido Eytel, Vientos de silencio (1999) by J.J. Faundes, and El lento silbido de los sables (2010) by Patricio Manns. I will show that fiction, even in validating indigenous justice claims, does not overcome past narratives of dominance. Representations can deceive us. The Mapuche warriors of Alonso de Ercilla’s epic poem La Araucana are safely in the past, and their cultural difference poses no threat to present-day nor- mative Chilean culture. However, that normative culture glorifies these fictional representations on the one hand and deprecates Mapuche communities today on the other. How did that hap- pen? Various Mapuche groups resisted Spanish colonization for centuries, restricting European expansion in today’s southern Chile. After Chilean independence from Spain in the early nine- teenth century, the conflict continued. -
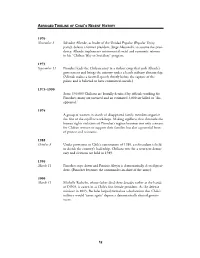
Abridged Timeline of Chile's Recent History
ABRIDGED TIMELINE OF CHILE’S RECENT HISTORY 1970 November 3 Salvador Allende, as leader of the Unidad Popular (Popular Unity party), defeats a former president, Jorge Alessandri, to assume the presi- dency. Allende implements controversial social and economic reforms in his “Chilean Way to Socialism” program. 1973 September 11 Pinochet leads the Chilean army in a violent coup that ends Allende’s government and brings the country under a harsh military dictatorship. (Allende makes a farewell speech shortly before the capture of the palace and is believed to have committed suicide.) 1973–1990 Some 130,000 Chileans are brutally detained by officials working for Pinochet; many are tortured and an estimated 3,000 are killed or “dis- appeared.” 1974 A group of women in search of disappeared family members organize the first of the arpillera workshops. Making arpilleras that chronicle the human rights violations of Pinochet’s regime becomes not only a means for Chilean women to support their families but also a powerful form of protest and resistance. 1988 October 5 Under provisions in Chile’s constitution of 1980, a referendum is held to decide the country’s leadership. Chileans vote for a return to democ- racy and elections are held in 1989. 1990 March 11 Pinochet steps down and Patricio Alwyn is democratically elected presi- dent. (Pinochet becomes the commander-in-chief of the army.) 2006 March 11 Michelle Bachelet, whose father died three decades earlier at the hands of DINA, is sworn in as Chile’s first female president. As the defense minister in 2003, Bachelet helped formulate a declaration that Chile’s military would “never again” depose a democratically elected govern- ment. -

Tourism in Chile
PK-6 THE AMERICAS Peter Keller is a Forest & Society Fellow of the Institute, studying and writing about national ICWA and private parks in Chile and Argentina. LETTERS Tourism in Chile By Peter Keller Since 1925 the Institute of September, 2000 Current World Affairs (the Crane- PUERTO VARAS, Chile – Chileans have a “secret” they are keeping from the rest Rogers Foundation) has provided of the world. Although some in Chile are trying to let the information out, most long-term fellowships to enable are unknowingly continuing this “joke” they have played upon the other conti- outstanding young professionals nents. If I didn’t know any better, I would think I could hear the sounds of ner- to live outside the United States vous giggles, the kind made by school children playing “hide and seek” just before and write about international being sought. We in North America and Europe have been duped into thinking areas and issues. An exempt all of South America — or Latin America, for that matter — is not worth our time, operating foundation endowed by especially our cherished vacations. We tend to lump all of the countries of South the late Charles R. Crane, the America together and think of them as being equal in the level of crime, poverty, Institute is also supported by pollution and general chaos. contributions from like-minded individuals and foundations. However, two countries stand apart from this stereotype, Argentina and Chile. A tourist visiting either of these countries is likely to think they are in Europe rather than South America due to the ease of travel (excellent bus systems), com- TRUSTEES fortable lodging and sanitary conditions (water safe to drink straight from the Carole Beaulieu tap, which I wouldn’t advise elsewhere on this continent). -

World Bank Document
Afro-descendants in Latin America Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Toward aFrameworkofInclusion in LatinAmerica Afro-descendants Afro-descendants in Latin America Toward a Framework of Inclusion Prepared by: Germán Freire Carolina Díaz-Bonilla Steven Schwartz Orellana Jorge Soler López Flavia Carbonari Latin America and the Caribbean Region Social, Urban, Rural and Resilience Global Practice Poverty and Equity Global Practice Afro-descendants in Latin America Toward a Framework of Inclusion Prepared by: Germán Freire Carolina Díaz-Bonilla Steven Schwartz Orellana Jorge Soler López Flavia Carbonari Latin America and the Caribbean Region Social, Urban, Rural and Resilience Global Practice Poverty and Equity Global Practice © 2018 International Bank for Reconstruction and Development / The World Bank 1818 H Street NW Washington DC 20433 Telephone: 202-473-1000 Internet: www.worldbank.org This work was originally published by The World Bank in English as Afro-descendants in Latin America: Toward a Framework of Inclusion, in 2018. In case of any discrepancies, the original language will prevail. This work is a product of the staff of The World Bank with external contributions. The findings, interpretations, and conclusions expressed in this work do not necessarily reflect the views of The World Bank, its Board of Executive Directors, or the governments they represent. The World Bank does not guarantee the accuracy of the data included in this work. The boundaries, colors, denominations, and other information shown on any map in this work do not imply any judgment on the part of The World Bank concerning the legal status of any territory or the endorsement or acceptance of such boundaries. -

Significados Que Los Sujetos De Intervención Le Atribuyen a La Relación Profesional Desarrollada Con Trabajadores Sociales
Significados que los Contesting stigma: sujetos de intervención afro-descendant le atribuyen a la migrants in Santiago, relación profesional Chile / Impugnando desarrollada con el estigma: migrantes trabajadores sociales afro-descendientes en CARMEN GLORIA JARPA Santiago de Chile. PAMELA CASTILLO MELISSA M. VALLE KAREN TORO Trabajo social chileno Aculturación en las y dictadura militar. ciencias sociales: Memoria profesional y la división del trabajo de las prácticas de olvido disciplinas sociales en la PATRICIA CASTAÑEDA MENESES política pública ANA MARÍA SALAMÉ COULON LUIS SARMIENTO Ética para la intervención social. Los valores aportados por el Trabajo Social y las éticas del cuidado y no paternalista como modelos de referencia para la práctica profesional CARLA CUBILLOS VEGA DICIEMBRE 20 87 14 ISSN 0716-9736 / Revista Trabajo Social / No 87 / Diciembre 2014 Afro-Descendant Migrants in Santiago, Chile: Stigma Processes and Rhetorical Resistance MELISSA M. VALLE Ph.D. Candidate Columbia University. Department of Sociology- Knox Hall 606 W. 122nd Street New York, New York 10027; [email protected] Summary This exploratory study seeks to demonstrate the mechanisms which lead to reduced life chances for marginalized groups, as well as to understand how they negotiate stigma perspectives that suggest their identities have been devalued. It provides a qualitative empirical account of the experiences of Afro-descendants presently living in Santiago, Chile, and contributes to the de- bate on the realities of migrants in Latin America from the perspective of an understudied, often marginalized and excluded population. Forty-eight semi-structured interviews were conducted with adult migrants of visibly of African-descent (27 female, 22 male) from 4 continents and 15 countries, between April and May 2013. -

The Mapuche People: Between Oblivion and Exclusion
n°358/2 August 2003 International Federation for Human Rights Report International Investigative Mission CHILE THE MAPUCHE PEOPLE: BETWEEN OBLIVION AND EXCLUSION I. INTRODUCTION AND PRESENTATION OF THE MISSION . 4 II. CONTEXT . 6 III. FORESTRY EXPLOITATION: THE DESTRUCTION OF A PEOPLE AND THEIR ENVIRONMENT. 10 IV. THE RALCO PROJECT: THE RESISTANCE OF A PEOPLE . 24 V. CONCLUSIONS AND RECOMMENDATIONS. 41 VI. APPENDIX . 45 VI. BIBLIOGRAPHY . 49 CHILE THE MAPUCHE PEOPLE: BETWEEN OBLIVION AND EXCLUSION INDEX I. INTRODUCTION AND PRESENTATION OF THE MISSION . 4 II. CONTEXT . 6 1. GENERAL FACTS ON THE INDIGENOUS POPULATION . 6 2. THE RIGHTS OF INDIGENOUS PEOPLES IN CHILE . 8 III. FORESTRY EXPLOITATION: THE DESTRUCTION OF A PEOPLE AND THEIR ENVIRONMENT. 10 1. HISTORICAL BACKGROUND AND ORIGIN OF THE CURRENT CONFLICT. 10 2. REPRESSION OF THE MAPUCHE PEOPLE . 13 3. JUDICIAL PERSECUTION OF LEADERS AND MEMBERS WITHIN THE MAPUCHE COMMUNITIES. 16 4. OTHER CONSEQUENCES OF FORESTRY EXPLOITATION ON THE MAPUCHE PEOPLE. 19 IV. THE RALCO PROJECT: THE RESISTANCE OF A PEOPLE . 24 1. BACKGROUND ON THE RALCO HYDROELECTRIC POWER PLANT . 25 a) Endesa's Mega-Hydraulic Project: Technical and Financial Aspects b) The Pangue Plant, the Pehuén Foundation and Downing-Hair Reports 2. IRREGULARITIES OF FORM AND SUBSTANCE IN THE RALCO CONCESSION . 27 a) Environmental Authorization: The Agreement Between ENDESA and CONOMA b) The Exchange of Pehuenche Land and CONADI's Authorization c) The Illegal Electrical Concession of The Ralco project 3. EFFECTS OF THE RALCO CONSTRUCTION ON THE PEHUENCHES. 34 a) Represssion of the Affected Communities b) The PehuencheS Women4S Resistance V. CONCLUSIONS AND RECOMMENDATIONS. 41 VI. -

The Role of Food: Culture in Health by Mary Schneider, Heather Heidenreich, Courtney Klappenbach, Alida Melse
1 The Role of Food: Culture in Health By Mary Schneider, Heather Heidenreich, Courtney Klappenbach, Alida Melse Abstract The central motivation for this research project was inspired by the rapidly increasing prevalence of obesity and diabetes around the globe. This led us to the evaluation of four coastal countries, including Japan, Greece, Spain, and Chile, and their relationship with diabetes and obesity. Our central question involving these countries was: what factors relating to culture contribute to possible predictions for health? We answered this question by evaluating several possible factors relating to each country including the physical environment, geography, types of food eaten, amount of processed food consumed, and the food preparation as possible predictors for health within these cultures. We explored these factors through methods using online databases including the Human Relations Area Files (HRAF), Organisation for Economic Co-operation and Development (OECD), and World Bank. We also utilized resources such as the WSU library and search engines to discover peer reviewed journal articles. As a result of this, we found that the environment and geography were not adequate predictors of health and culture due to the fact that all our countries were similar in geography and still had various health results. We then found that the types of food consumed were also not adequate predictors of health related to culture since there was significant overlap in the types of food used in these countries. This led to the conclusion that when evaluating the culture around food in four developed nations, the most prevalent food-related indicator of health was the level of food processing and preparation. -

A Brief History of Araucanian Studies Donald Brand
New Mexico Anthropologist Volume 5 | Issue 2 Article 2 6-1-1941 A Brief History of Araucanian Studies Donald Brand Follow this and additional works at: https://digitalrepository.unm.edu/nm_anthropologist Recommended Citation Brand, Donald. "A Brief History of Araucanian Studies." New Mexico Anthropologist 5, 2 (1941): 19-35. https://digitalrepository.unm.edu/nm_anthropologist/vol5/iss2/2 This Article is brought to you for free and open access by the Anthropology at UNM Digital Repository. It has been accepted for inclusion in New Mexico Anthropologist by an authorized editor of UNM Digital Repository. For more information, please contact [email protected]. A BRIEF HISTORY OF ARAUCANIAN STUDIES By DONALD D. BRAND The term Araucanian most properly refers to the language once spoken by the many Indian groups between the Rio Choapa (Coquimbo Province) and the Gulf of Corcovado (Chiloe Province). However, growing usage-both vulgar and scientific-makes advisable the use of this name for the Indians themselves, although they were never a political, physical, or cultural unit. Probably the first European contact with the Araucanians was in 1536, when some of Diego de Almagro's scouts advanced into central Chile. However, it remained for Pedro de Valdivia, in 1541, to con- quer all of Araucanian Chile earlier held by the Incas (to the Rio Maule), and to push southward across the Rio Bio-Bio into the forest home of the unconquered Araucanians. Here, in 1553, Valdivia lost his life to these Indians-led by Lautaro and Caupolicdn. The con- quest was continued by Garcia Hurtado de Mendoza, in whose small army was Alonso de Ercilla y Zdfiiga (Madrid 1533-1594 Madrid). -

Tourism in Chile
WWW . INVESTCHILE . GOB . CL IN CHILE TOURISM OPPORTUNITIES INVESTMENT MAP OF FOTOGRAFÍA TURISMO CHILE TURISMO FOTOGRAFÍA CHILE IN TOURISM A PARTNER IN FOR YOUR OPPORTUNITIES INVESTMENT INVESTMENT OF MAP , Chile is the best evaluated economy in Latin America and, indeed, one of the best evaluated emerging economies worldwide. TOURISM Its hallmark stability, transparency and competitiveness and excellent business prospects position it not only as the best destination for foreign investment in Latin America but also as one of the most outstanding in the world. In its World Investment Report 2015, UNCTAD ranked Chile as the world’s 11th largest recipient of foreign direct investment (FDI), with an inflow that reached US$20,457 million. FDI in Chile 2004-2015 (US$ million) *Source: Central Bank of Chile 28.45728,457 23.30923,309 22.00222,002 20.45720,457 19.26419,264 CL 16.60416,604 . 15.51015,510 13.17813,178 13.39213,392 GOB . 8.7988,798 7.2417,241 7.4827,482 INVESTCHILE . WWW 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 FOREIGN INVESTMENT PROMOTION AGENCY FOTOGRAFÍA TURISMO CHILE TURISMO FOTOGRAFÍA FROMEN CHILE CHILE Overseas companies have reduced- tariff access to markets that account for 85% of global GDP. 3 CHILE IN TOURISM IN OPPORTUNITIES INVESTMENT OF MAP , WWW . INVESTCHILE . GOB . CL TOURISM , MAP OF INVESTMENT OPPORTUNITIES IN TOURISM IN CHILE TOURISM WHY IN INVEST CHILE OPEN ECONOMY OPEN CL . GOB . LOW RISK LEADER INVESTCHILE . ? WWW FOREIGN INVESTMENT PROMOTION AGENCY BECAUSE IT LEADS LATIN AMERICA IN Business