Development of a Small Panel of Snps to Infer Ancestry in Chileans That Distinguishes Aymara and Mapuche Components Ricardo A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

By Amalia Damgaard
By Private Chef Amalia Damgaard CHILEAN PANORAMA Although it appears slim and small, Chile is a long and narrow country about the size of Texas, with a vast coast line covering about 3,998 miles. The Pacific Ocean borders to the west; Argentina is a neighbor to the east; Bolivia, to the northeast; and Peru, to the north. Because of its geographical location, Chile has an unusual and fun landscape, with deserts, beaches, fjords, glaciers and icebergs, fertile lands, the Andes mountains, over 600 volcanoes (some active), and sub-artic conditions in the South. Since Chile is below the equator, their seasons are different from ours in the United States. So, when we have winter they have summer, and so on. Even though Chile had years of political and economic turmoil, it has evolved into a market-oriented economy with strong foreign trade. Currently, it has the strongest economy in South America, with a relatively-low crime rate, and a high standard of living. Chile is a land rich in beauty, culture, and literature. It is called “the Switzerland of South America” because of its natural splendor. World renowned poets, Pablo Neruda and Gabriela Mistral, won Nobel Prizes. The majority of Chileans are descendants of Europeans, namely Spanish, French, and German, and others in smaller numbers. Allegedly, the original inhabitants of the region prior to Spanish conquest were not natives but merely nomads who lived in the area. Their descendants are today about 3% of the population. A mixture of the so-called natives and European settlers is called “mestizo.” Today’s mestizos are so well blended that they look mostly European. -

SURNAMES in CHILE a Study of the Population of Chile Through
Page 1 of 31 American Journal of Physical Anthropology 1 2 3 SURNAMES IN CHILE 4 5 A study of the population of Chile through isonymy 6 I. Barrai, A. Rodriguez-Larralde 2, J. Dipierri 1, E.Alfaro 1, N. Acevedo 3, 7 8 E. Mamolini, M. Sandri, A.Carrieri and C. Scapoli. 9 10 Dipartimento di Biologia ed Evoluzione, Università di Ferrara, 44121- Ferrara, Italy 11 1Instituto de Biología de la Altura, Universidad Nacional de Jujuy, 4600 – San Salvador De Jujuy, 12 13 Argentina. 14 2 15 Centro de Medicina Experimental, Laboratorio de Genetica Humana, IVIC, 1020A -Caracas, 16 Venezuela. 17 18 3Museo Nacional de Ciencias Naturales, Santiago, Chile 19 20 21 Running title: Surnames in Chile 22 23 24 25 26 Correspondence to: 27 Chiara Scapoli 28 Department of Biology and Evolution 29 30 University of Ferrara, 31 Via L. Borsari 46, - I-44121 Ferrara, Italy. 32 Telephone: +39 0532 455744; FAX: : +39 0532 249761 33 Email: [email protected] 34 35 36 Number of text pages: 15 37 Literature pages: 4 38 39 Number of Tables : 2 40 41 Number of Figures: 7 42 43 44 KEYWORDS : Chile, Population Structure, Isonymy, Inbreeding, Isolation by distance 45 46 47 ACKNOWLEDGMENTS: The authors are grateful to the Director of the Servicio Electoral de la 48 49 Republica de Chile Sr. Juan Ignacio Garcia Rodríguez, who made the data available, and to Sr. 50 51 Dr.Ginés Mario Gonzalez Garcia, Embajador de la Republica Argentina en Chile. The work was 52 supported by grants of the Italian Ministry of Universities and Research (MIUR) to Chiara Scapoli. -

Permanent War on Peru's Periphery: Frontier Identity
id2653500 pdfMachine by Broadgun Software - a great PDF writer! - a great PDF creator! - http://www.pdfmachine.com http://www.broadgun.com ’S PERIPHERY: FRONT PERMANENT WAR ON PERU IER IDENTITY AND THE POLITICS OF CONFLICT IN 17TH CENTURY CHILE. By Eugene Clark Berger Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in History August, 2006 Nashville, Tennessee Approved: Date: Jane Landers August, 2006 Marshall Eakin August, 2006 Daniel Usner August, 2006 íos Eddie Wright-R August, 2006 áuregui Carlos J August, 2006 id2725625 pdfMachine by Broadgun Software - a great PDF writer! - a great PDF creator! - http://www.pdfmachine.com http://www.broadgun.com HISTORY ’ PERMANENT WAR ON PERU S PERIPHERY: FRONTIER IDENTITY AND THE POLITICS OF CONFLICT IN 17TH-CENTURY CHILE EUGENE CLARK BERGER Dissertation under the direction of Professor Jane Landers This dissertation argues that rather than making a concerted effort to stabilize the Spanish-indigenous frontier in the south of the colony, colonists and indigenous residents of 17th century Chile purposefully perpetuated the conflict to benefit personally from the spoils of war and use to their advantage the resources sent by viceregal authorities to fight it. Using original documents I gathered in research trips to Chile and Spain, I am able to reconstruct the debates that went on both sides of the Atlantic over funds, protection from ’ th pirates, and indigenous slavery that so defined Chile s formative 17 century. While my conclusions are unique, frontier residents from Paraguay to northern New Spain were also dealing with volatile indigenous alliances, threats from European enemies, and questions about how their tiny settlements could get and keep the attention of the crown. -

THE PEOPLES and LANGUAGES of CHILE by DONALDD
New Mexico Anthropologist Volume 5 | Issue 3 Article 2 9-1-1941 The eoplesP and Languages of Chile Donald Brand Follow this and additional works at: https://digitalrepository.unm.edu/nm_anthropologist Recommended Citation Brand, Donald. "The eP oples and Languages of Chile." New Mexico Anthropologist 5, 3 (1941): 72-93. https://digitalrepository.unm.edu/nm_anthropologist/vol5/iss3/2 This Article is brought to you for free and open access by the Anthropology at UNM Digital Repository. It has been accepted for inclusion in New Mexico Anthropologist by an authorized editor of UNM Digital Repository. For more information, please contact [email protected]. 72 NEW MEXICO ANTHROPOLOGIST THE PEOPLES AND LANGUAGES OF CHILE By DONALDD. BRAND This article initiates a series in which the writer will attempt to summarize the scattered and commonly contradictory material on the present ethnic and linguistic constituency of a number of Latin Ameri- can countries. It represents some personal investigations in the field and an examination of much of the pertinent literature. Chile has been a sovereign state since the War of Independence 1810-26. This state was founded upon a nuclear area west of the Andean crest and essentially between 240 and 460 South Latitude. Through the War of the Pacific with Bolivia and Perui in 1879-1883 and peaceful agreements with Argentina, Chile acquired her present extention from Arica to Tierra del Fuego. These northern and south- ern acquisitions added little to her population but introduced numerous small ethnic and linguistic groups. Chile has taken national censuses in 1835, 1843, 1854, 1865, 1875, 1885, 1895, 1907, 1920, 1930, and the most recent one in November of 1940. -

Aspects of Social Justice Ally Work in Chilean Historical Fiction: the Case of the Pacification of Araucanía
Aspects of Social Justice Ally Work in Chilean Historical Fiction: The Case of the Pacification of Araucanía Katherine Karr-Cornejo Whitworth University Abstract Majority-culture writers often depict cultures different from their own, but approaching cultures to which an author does not belong can be challenging. How might we read dominant-culture portrayals of marginalized cultures that tell stories of injustice? In this paper I utilize the frame of identity development in social justice allies in order to understand the narratives dominant-culture authors use in fiction to reflect sympathetic views of indigenous justice claims. In order to do so, I study three historical novels set in Araucanía during the second half of the nineteenth century, considering historiographical orientation, representation of cultural difference, and understanding of sovereignty:Casas en el agua (1997) by Guido Eytel, Vientos de silencio (1999) by J.J. Faundes, and El lento silbido de los sables (2010) by Patricio Manns. I will show that fiction, even in validating indigenous justice claims, does not overcome past narratives of dominance. Representations can deceive us. The Mapuche warriors of Alonso de Ercilla’s epic poem La Araucana are safely in the past, and their cultural difference poses no threat to present-day nor- mative Chilean culture. However, that normative culture glorifies these fictional representations on the one hand and deprecates Mapuche communities today on the other. How did that hap- pen? Various Mapuche groups resisted Spanish colonization for centuries, restricting European expansion in today’s southern Chile. After Chilean independence from Spain in the early nine- teenth century, the conflict continued. -
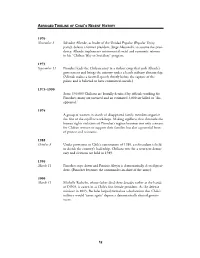
Abridged Timeline of Chile's Recent History
ABRIDGED TIMELINE OF CHILE’S RECENT HISTORY 1970 November 3 Salvador Allende, as leader of the Unidad Popular (Popular Unity party), defeats a former president, Jorge Alessandri, to assume the presi- dency. Allende implements controversial social and economic reforms in his “Chilean Way to Socialism” program. 1973 September 11 Pinochet leads the Chilean army in a violent coup that ends Allende’s government and brings the country under a harsh military dictatorship. (Allende makes a farewell speech shortly before the capture of the palace and is believed to have committed suicide.) 1973–1990 Some 130,000 Chileans are brutally detained by officials working for Pinochet; many are tortured and an estimated 3,000 are killed or “dis- appeared.” 1974 A group of women in search of disappeared family members organize the first of the arpillera workshops. Making arpilleras that chronicle the human rights violations of Pinochet’s regime becomes not only a means for Chilean women to support their families but also a powerful form of protest and resistance. 1988 October 5 Under provisions in Chile’s constitution of 1980, a referendum is held to decide the country’s leadership. Chileans vote for a return to democ- racy and elections are held in 1989. 1990 March 11 Pinochet steps down and Patricio Alwyn is democratically elected presi- dent. (Pinochet becomes the commander-in-chief of the army.) 2006 March 11 Michelle Bachelet, whose father died three decades earlier at the hands of DINA, is sworn in as Chile’s first female president. As the defense minister in 2003, Bachelet helped formulate a declaration that Chile’s military would “never again” depose a democratically elected govern- ment. -

Tourism in Chile
PK-6 THE AMERICAS Peter Keller is a Forest & Society Fellow of the Institute, studying and writing about national ICWA and private parks in Chile and Argentina. LETTERS Tourism in Chile By Peter Keller Since 1925 the Institute of September, 2000 Current World Affairs (the Crane- PUERTO VARAS, Chile – Chileans have a “secret” they are keeping from the rest Rogers Foundation) has provided of the world. Although some in Chile are trying to let the information out, most long-term fellowships to enable are unknowingly continuing this “joke” they have played upon the other conti- outstanding young professionals nents. If I didn’t know any better, I would think I could hear the sounds of ner- to live outside the United States vous giggles, the kind made by school children playing “hide and seek” just before and write about international being sought. We in North America and Europe have been duped into thinking areas and issues. An exempt all of South America — or Latin America, for that matter — is not worth our time, operating foundation endowed by especially our cherished vacations. We tend to lump all of the countries of South the late Charles R. Crane, the America together and think of them as being equal in the level of crime, poverty, Institute is also supported by pollution and general chaos. contributions from like-minded individuals and foundations. However, two countries stand apart from this stereotype, Argentina and Chile. A tourist visiting either of these countries is likely to think they are in Europe rather than South America due to the ease of travel (excellent bus systems), com- TRUSTEES fortable lodging and sanitary conditions (water safe to drink straight from the Carole Beaulieu tap, which I wouldn’t advise elsewhere on this continent). -

World Bank Document
Afro-descendants in Latin America Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Toward aFrameworkofInclusion in LatinAmerica Afro-descendants Afro-descendants in Latin America Toward a Framework of Inclusion Prepared by: Germán Freire Carolina Díaz-Bonilla Steven Schwartz Orellana Jorge Soler López Flavia Carbonari Latin America and the Caribbean Region Social, Urban, Rural and Resilience Global Practice Poverty and Equity Global Practice Afro-descendants in Latin America Toward a Framework of Inclusion Prepared by: Germán Freire Carolina Díaz-Bonilla Steven Schwartz Orellana Jorge Soler López Flavia Carbonari Latin America and the Caribbean Region Social, Urban, Rural and Resilience Global Practice Poverty and Equity Global Practice © 2018 International Bank for Reconstruction and Development / The World Bank 1818 H Street NW Washington DC 20433 Telephone: 202-473-1000 Internet: www.worldbank.org This work was originally published by The World Bank in English as Afro-descendants in Latin America: Toward a Framework of Inclusion, in 2018. In case of any discrepancies, the original language will prevail. This work is a product of the staff of The World Bank with external contributions. The findings, interpretations, and conclusions expressed in this work do not necessarily reflect the views of The World Bank, its Board of Executive Directors, or the governments they represent. The World Bank does not guarantee the accuracy of the data included in this work. The boundaries, colors, denominations, and other information shown on any map in this work do not imply any judgment on the part of The World Bank concerning the legal status of any territory or the endorsement or acceptance of such boundaries. -

The Localization of the Global Agendas How Local Action Is Transforming Territories and Communities
2019 The Localization of the Global Agendas How local action is transforming territories and communities Fifth Global Report on Decentralization and Local Democracy 2 GOLD V REPORT © 2019 UCLG The right of UCLG to be identified as author of the editorial material, and of the individual authors as authors of their contributions, has been asserted by them in accordance with sections 77 and 78 of the Copyright, Designs and Patents Act 1988. All rights reserved. No part of this book may be reprinted or reproduced or utilized in any form or by any electronic, mechanical or other means, now known or hereafter invented, including photocopying and recording, or in any information storage or retrieval system, without permission in writing from the publishers. United Cities and Local Governments Cités et Gouvernements Locaux Unis Ciudades y Gobiernos Locales Unidos Avinyó 15 08002 Barcelona www.uclg.org DISCLAIMERS The terms used concerning the legal status of any country, territory, city or area, or of its authorities, or concerning delimitation of its frontiers or boundaries, or regarding its economic system or degree of development do not necessarily reflect the opinion of United Cities and Local Governments. The analysis, conclusions and recommendations of this report do not necessarily reflect the views of all the members of United Cities and Local Governments. This document has been produced with the financial assistance of the European Union. The contents of this document are the sole responsibility of UCLG and can under no circumstances be regarded as reflecting the position of the European Union. Graphic design and lay-out: www.ggrafic.com Cover photos: A-C-K (t.ly/xP7pw); sunriseOdyssey (bit.ly/2ooZTnM); TEDxLuanda (bit.ly/33eFEIt); Curtis MacNewton (bit.ly/2Vm5Yh1); s1ingshot (t.ly/yWrwV); Chuck Martin (bit.ly/30ReOEz); sunsinger (shutr.bz/33dH85N); Michael Descharles (t.ly/Mz7w3). -

Revista Latinoamericana De Investigación Crítica Año IV Nº 6 | Publicación Semestral | Enero-Junio De 2017
Introducción CARLOS FIDEL Revista latinoamericana TEMA CENTRAL: LAS RELIGIONES SON UN MUNDO EN AMÉRICA LATINA de investigación crítica Éticas, afinidades, aversiones y doctrinas: capitalismos y cristianismos en América Latina. Relectura a partir de Max Weber ISSN 2409-1308 - Año IV Nº6 y Ernst Troelstch FORTUNATO MALLIMACI Enero - Junio 2017 Movilización política, memoria y simbología religiosa: San Cayetano 6 y los movimientos sociales en Argentina VERÓNICA GIMÉNEZ BÉLIVEAU Y MARCOS ANDRÉS CARBONELLI Entrevista a ÁLVARO GARCÍA LINERA Religiones, cambio climático y transición hacia energías renovables: estudios recientes en Chile CRISTIÁN PARKER GUMUCIO Autoridad y lo común en procesos de minoritización: el pentecostalismo brasileño JOANILDO BURITY OTRAS TEMÁTICAS Acerca de los Derechos Culturales MARÍA VICTORIA ALONSO Y DIEGO FIDEL Los intentos de cambio ante la inercia de los sistemas policiales y jurídicos en las nuevas democracias SUSANA MALLO APORTES DE COYUNTURA “Argentina: ¿hacia dónde vamos?” ALDO FERRER ENTREVISTAS Álvaro García Linera “La gente no se mueve solo porque sufre” MARTÍN GRANOVSKY FORTUNATO MALLIMACI VERÓNICA GIMÉNEZ BÉLIVEAU SOCIEDAD Y ARTES MARCOS ANDRÉS CARBONELLI “Las paradojas de Quiriguá” LEANDRO KATZ Y JESSE LERNER CRISTIÁN PARKER GUMUCIO JOANILDO BURITY MARÍA VICTORIA ALONSO DIEGO FIDEL SUSANA MALLO ALDO FERRER LEANDRO KATZ JESSE LERNER ISSN 2409-1308 crítica latinoamericana de investigación Revista Fotos: “Las paradojas de Quiriguá” LEANDRO KATZ 9 772409 130008 6 Revista latinoamericana de investigación crítica -

Identifying Hotspots of Cardiometabolic Outcomes Based on a Bayesian Approach: the Example of Chile
PLOS ONE RESEARCH ARTICLE Identifying hotspots of cardiometabolic outcomes based on a Bayesian approach: The example of Chile 1 2 1 3 Gloria A. AguayoID *, Anna Schritz , Maria Ruiz-Castell , Luis Villarroel , 3 1 4,5 6 Gonzalo Valdivia , Guy FagherazziID , Daniel R. WitteID , Andrew Lawson 1 Population Health Department, Luxembourg Institute of Health, Strassen, Luxembourg, 2 Competence Center for Methodology and Statistics, Luxembourg Institute of Health, Strassen, Luxembourg, 3 Department of Public Health, School of Medicine, Pontificia Universidad CatoÂlica de Chile, Santiago, Chile, 4 Department of Public Health, Aarhus University, Aarhus, Denmark, 5 Danish Diabetes Academy, Odense, Denmark, a1111111111 6 Department of Public Health Sciences, Medical University of South Carolina, South Carolina, Charleston, a1111111111 United States of America a1111111111 * [email protected] a1111111111 a1111111111 Abstract OPEN ACCESS Background Citation: Aguayo GA, Schritz A, Ruiz-Castell M, There is a need to identify priority zones for cardiometabolic prevention. Disease mapping in Villarroel L, Valdivia G, Fagherazzi G, et al. (2020) countries with high heterogeneity in the geographic distribution of the population is challeng- Identifying hotspots of cardiometabolic outcomes based on a Bayesian approach: The example of ing. Our goal was to map the cardiometabolic health and identify hotspots of disease using Chile. PLoS ONE 15(6): e0235009. https://doi.org/ data from a national health survey. 10.1371/journal.pone.0235009 Editor: Nayu Ikeda, National Institute of Health and Methods Nutrition, National Institutes of Biomedical Innovation, Health and Nutrition, JAPAN Using Chile as a case study, we applied a Bayesian hierarchical modelling. We performed a Received: December 10, 2019 cross-sectional analysis of the 2009±2010 Chilean Health Survey. -

Achieving Educational Quality: What Schools Teach Us. Learning From
64 S E R I E desarrollo productivo Achieving educational quality: What schools teach us Learning from Chile’s P900 primary schools Beverley A. Carlson Restructuring and Competitiveness Network Division of Production, Productivity and Management Santiago, Chile, January, 2000 This document was prepared by Ms. Beverley A. Carlson (e-mail: [email protected]), Social Affairs Officer, Division of Production, Productivity and Management of the Economic Commission for Latin America and the Caribbean of the United Nations (ECLAC). The views expressed in this document, which has been reproduced without formal editing, are those of the author and do not necessarily reflect the views of the Organization. The author would like to express gratitude to the Ministry of Education of Chile and its P900 schools programme, provincial authorities and, especially, each of the schools in the study that shared their time, energy and knowledge, and are the center of this work. The author would also like to thank the following people for their support in carrying out the study: Pilar Bascuñán, David Cornejo, Françoise Delannoy, Juan Eduardo García-Huidobro, Lysette Henríquez, Mónica Jaramillo, Howard LaFranchi, Laura López, Elizabeth Love, Rosalía Manchego, Iván Ortiz, Isolda Pacheco, Walter Parraguez, Joseph Ramos, Juan Serrano, Alejandra Silva and Carmen Sotomayor. United Nations Publication LC/L.1279-P ISSN: 1020-5179 ISBN: 92-1-121249-9 Copyright © United Nations, January, 2000. All rights reserved Sales N°: E.99.II.G.60 Printed in United Nations, Santiago, Chile Applications for the right to reproduce this work are welcomed and should be sent to the Secretary of the Publications Board, United Nations Headquarters, New York, N.Y.