Components of Endocannabinoid Signaling System Are Expressed in the Perinatal Mouse Cerebellum and Required for Its Normal Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Brainstem: Midbrainmidbrain
Brainstem:Brainstem: MidbrainMidbrain 1.1. MidbrainMidbrain –– grossgross externalexternal anatomyanatomy 2.2. InternalInternal structurestructure ofof thethe midbrain:midbrain: cerebral peduncles tegmentum tectum (guadrigeminal plate) Midbrain MidbrainMidbrain –– generalgeneral featuresfeatures location – between forebrain and hindbrain the smallest region of the brainstem – 6-7g the shortest brainstem segment ~ 2 cm long least differentiated brainstem division human midbrain is archipallian – shared general architecture with the most ancient of vertebrates embryonic origin – mesencephalon main functions:functions a sort of relay station for sound and visual information serves as a nerve pathway of the cerebral hemispheres controls the eye movement involved in control of body movement Prof. Dr. Nikolai Lazarov 2 Midbrain MidbrainMidbrain –– grossgross anatomyanatomy dorsal part – tectum (quadrigeminal plate): superior colliculi inferior colliculi cerebral aqueduct ventral part – cerebral peduncles:peduncles dorsal – tegmentum (central part) ventral – cerebral crus substantia nigra Prof. Dr. Nikolai Lazarov 3 Midbrain CerebralCerebral cruscrus –– internalinternal structurestructure CerebralCerebral peduncle:peduncle: crus cerebri tegmentum mesencephali substantia nigra two thick semilunar white matter bundles composition – somatotopically arranged motor tracts: corticospinal } pyramidal tracts – medial ⅔ corticobulbar corticopontine fibers: frontopontine tracts – medially temporopontine tracts – laterally -

Lecture 12 Notes
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -

Functional Neuroanatomy for Posture and Gait Control
REVIEW ARTICLE https://doi.org/10.14802/jmd.16062 / J Mov Disord 2017;10(1):1-17 pISSN 2005-940X / eISSN 2093-4939 Functional ABSTRACT Here we argue functional neuroanatomy for pos- ture-gait control. Multi-sensory information such as Neuroanatomy somatosensory, visual and vestibular sensation act on various areas of the brain so that adaptable pos- ture-gait control can be achieved. Automatic process for Posture and of gait, which is steady-state stepping movements associating with postural reflexes including head- eye coordination accompanied by appropriate align- Gait Control ment of body segments and optimal level of pos- tural muscle tone, is mediated by the descending pathways from the brainstem to the spinal cord. Par- ticularly, reticulospinal pathways arising from the lat- Kaoru Takakusaki eral part of the mesopontine tegmentum and spinal locomotor network contribute to this process. On The Research Center for Brain Function and Medical Engineering, the other hand, walking in unfamiliar circumstance Asahikawa Medical University, Asahikawa, Japan requires cognitive process of postural control, which depends on knowledges of self-body, such as body schema and body motion in space. The cognitive in- formation is produced at the temporoparietal asso- ciation cortex, and is fundamental to sustention of vertical posture and construction of motor programs. The programs in the motor cortical areas run to ex- ecute anticipatory postural adjustment that is opti- mal for achievement of goal-directed movements. The basal ganglia and cerebellum may affect both the automatic and cognitive processes of posture- gait control through reciprocal connections with the brainstem and cerebral cortex, respectively. Conse- quently, impairments in cognitive function by dam- ages in the cerebral cortex, basal ganglia and cere- bellum may disturb posture-gait control, resulting in falling. -

Compensatory Sprouting and Impulse Rerouting After Unilateral Pyramidal Tract Lesion in Neonatal Rats
The Journal of Neuroscience, September 1, 2000, 20(17):6561–6569 Compensatory Sprouting and Impulse Rerouting after Unilateral Pyramidal Tract Lesion in Neonatal Rats Werner J. Z’Graggen, Karim Fouad, Olivier Raineteau, Gerlinde A. S. Metz, Martin E. Schwab, and Gwendolyn L. Kartje Brain Research Institute, University of Zurich and Swiss Federal Institute of Technology Zurich, CH-8057 Zurich, Switzerland After lesions of the developing mammalian CNS, structural plas- tigate possible new functional connections from the motor cortex ticity and functional recovery are much more pronounced than in of the pyramidotomy side to the periphery. In rats lesioned as the mature CNS. We investigated the anatomical reorganization adults, stimulation of the motor cortex ipsilateral to the pyra- of the corticofugal projections rostral to a unilateral lesion of the midotomy never elicited EMG activity. In contrast, in P2 lesioned corticospinal tract at the level of the medullary pyramid (pyra- rats bilateral forelimb EMGs were found. EMG latencies were midotomy) and the contribution of this reorganization and other comparable for the ipsilateral and contralateral responses but descending systems to functional recovery. were significantly longer than in unlesioned animals. Transient Two-day-old (P2) and adult rats underwent a unilateral pyra- inactivation of both red nuclei with the GABA receptor agonist midotomy. Three months later the corticofugal projections to the muscimol led to a complete loss of these bilateral movements. red nucleus and the pons were analyzed; a relatively large num- Movements and EMGs reappeared after wash-out of the drug. ber of corticorubral and corticopontine fibers from the lesioned These results suggest an important role of the red nucleus in the side had crossed the midline and established an additional con- tralateral innervation of the red nucleus and the pons. -

Basal Ganglia and Cerebellum Receive Different Somatosensory Information in Rats (Barrel Field/Pontine Nuclei/Cortical Lamnation/Vibrissae) BARBARA E
Proc. Nati. Acad. Sci. USA Vol. 87, pp. 4388-4392, June 1990 Neurobiology Basal ganglia and cerebellum receive different somatosensory information in rats (barrel field/pontine nuclei/cortical lamnation/vibrissae) BARBARA E. MERCIER*, CHARLES R. LEGGt, AND MITCHELL GLICKSTEIN Department of Anatomy and Developmental Biology, University College London, Gower Street, London WC1, United Kingdom Communicated by Irving T. Diamond, March 14, 1990 ABSTRACT There are two great subcortical circuits that sublamina Vb stain far more densely, suggesting that they are relay sensory information to motor structures in the mamma- capable ofhigher levels oftonic activity than those in Va (12). lian brain. One pathway relays via the pontine nuclei and Previous study of the efferent pathways from the rat cerebellum, and the other relays by way of the basal ganglia. somatosensory cortex (2) has suggested that the cell popu- We studied the cells oforigin ofthese two major pathways from lation projecting to the basal ganglia is centered on lamina Va, the posteromedial barrel subfield ofrats, a distinct region ofthe while the population projecting to the cerebellum is centered somatosensory cortex that contains the sensory representation on Vb. However, the methods available at the time the study of the large whiskers. We itjected tracer substances into the was done were relatively insensitive (13) and a comparison caudate putamen or the pontine nuclei and charted the location with more recent work (14) shows that only a subset of the of retrogradely filled cortical cells. In preliminary studies, we entire population of corticopontine cells was identified. Wise used double-labeling techniques to determine whether the cells and Jones (figure 6a in ref. -

Chapter 3: Internal Anatomy of the Central Nervous System
10353-03_CH03.qxd 8/30/07 1:12 PM Page 82 3 Internal Anatomy of the Central Nervous System LEARNING OBJECTIVES Nuclear structures and fiber tracts related to various functional systems exist side by side at each level of the After studying this chapter, students should be able to: nervous system. Because disease processes in the brain • Identify the shapes of corticospinal fibers at different rarely strike only one anatomic structure or pathway, there neuraxial levels is a tendency for a series of related and unrelated clinical symptoms to emerge after a brain injury. A thorough knowl- • Recognize the ventricular cavity at various neuroaxial edge of the internal brain structures, including their shape, levels size, location, and proximity, makes it easier to understand • Recognize major internal anatomic structures of the their functional significance. In addition, the proximity of spinal cord and describe their functions nuclear structures and fiber tracts explains multiple symp- toms that may develop from a single lesion site. • Recognize important internal anatomic structures of the medulla and explain their functions • Recognize important internal anatomic structures of the ANATOMIC ORIENTATION pons and describe their functions LANDMARKS • Identify important internal anatomic structures of the midbrain and discuss their functions Two distinct anatomic landmarks used for visual orientation to the internal anatomy of the brain are the shapes of the • Recognize important internal anatomic structures of the descending corticospinal fibers and the ventricular cavity forebrain (diencephalon, basal ganglia, and limbic (Fig. 3-1). Both are present throughout the brain, although structures) and describe their functions their shape and size vary as one progresses caudally from the • Follow the continuation of major anatomic structures rostral forebrain (telencephalon) to the caudal brainstem. -

The Long Journey of Pontine Nuclei Neurons : from Rhombic Lip To
REVIEW Erschienen in: Frontiers in Neural Circuits ; 11 (2017). - 33 published: 17 May 2017 http://dx.doi.org/10.3389/fncir.2017.00033 doi: 10.3389/fncir.2017.00033 The Long Journey of Pontine Nuclei Neurons: From Rhombic Lip to Cortico-Ponto-Cerebellar Circuitry Claudius F. Kratochwil 1,2, Upasana Maheshwari 3,4 and Filippo M. Rijli 3,4* 1Chair in Zoology and Evolutionary Biology, Department of Biology, University of Konstanz, Konstanz, Germany, 2Zukunftskolleg, University of Konstanz, Konstanz, Germany, 3Friedrich Miescher Institute for Biomedical Research, Basel, Switzerland, 4University of Basel, Basel, Switzerland The pontine nuclei (PN) are the largest of the precerebellar nuclei, neuronal assemblies in the hindbrain providing principal input to the cerebellum. The PN are predominantly innervated by the cerebral cortex and project as mossy fibers to the cerebellar hemispheres. Here, we comprehensively review the development of the PN from specification to migration, nucleogenesis and circuit formation. PN neurons originate at the posterior rhombic lip and migrate tangentially crossing several rhombomere derived territories to reach their final position in ventral part of the pons. The developing PN provide a classical example of tangential neuronal migration and a study system for understanding its molecular underpinnings. We anticipate that understanding the mechanisms of PN migration and assembly will also permit a deeper understanding of the molecular and cellular basis of cortico-cerebellar circuit formation and function. Keywords: pontine gray nuclei, reticulotegmental nuclei, precerebellar system, cortico-ponto-cerebellar circuitry, Hox genes Edited by: Takao K. Hensch, INTRODUCTION Harvard University, United States Reviewed by: The basal pontine nuclei (BPN) (also known as basilar pons, pontine gray nuclei or pontine nuclei Masahiko Takada, (PN)) and the reticulotegmental nuclei (RTN) (also known as nucleus reticularis tegmenti pontis) Kyoto University, Japan are located within the ventral portion of the pons. -

Cerebral Cortex 595
Cerebral cortex 595 CEREBRAL CORTEX Gross Structure of Cerebral Hemispheres You have already looked at the blood supply of the cerebral hemispheres in lab, and will be dissecting and slicing the cerebral hemispheres to study deeper structures in a later lab. A detailed understanding of the blood supply of the cerebral cortex and its fiber systems is extremely important since blockage and rupture of these vessels accounts for a large proportion of the pathology of the brain. An extremely important point is that even though there usually are small anastomoses between branches of the different cerebral arteries or between cerebral arteries and dural arteries, these anastomoses are inadequate to supply sufficient blood to prevent loss of function. Typically, blockage of any one of the three arteries supplying the cerebral cortex (distal to their origin at the Circle of Willis) produces a rapid and irreversible destruction of a large region of cortex, although the exact extent of destruction is somewhat variable in different individuals as a result of differences in the extent of anastomoses. The pattern of distribution of the three arteries to the cerebral cortex (anterior, middle, and posterior cerebral) is seen in figures 2-4. The arterial supply for some of the more important parts of the cortex is as follows: Visual cortex (areas 17, 18, 19) in occipital lobe: posterior cerebral Somatic sensory (areas 3, 1, 2) and motor cortex (areas 4 & 6): anterior cerebral and middle cerebral. The foot and leg representations which are found medially are supplied by the anterior cerebral; the remainder of the body is supplied by the middle cerebral. -

White Matter Anatomy: What the Radiologist Needs to Know
White Matter Anatomy What the Radiologist Needs to Know Victor Wycoco, MBBS, FRANZCRa, Manohar Shroff, MD, DABR, FRCPCa,*, Sniya Sudhakar, MBBS, DNB, MDb, Wayne Lee, MSca KEYWORDS Diffusion tensor imaging (DTI) White matter tracts Projection fibers Association Fibers Commissural fibers KEY POINTS Diffusion tensor imaging (DTI) has emerged as an excellent tool for in vivo demonstration of white matter microstructure and has revolutionized our understanding of the same. Information on normal connectivity and relations of different white matter networks and their role in different disease conditions is still evolving. Evidence is mounting on causal relations of abnormal white matter microstructure and connectivity in a wide range of pediatric neurocognitive and white matter diseases. Hence there is a pressing need for every neuroradiologist to acquire a strong basic knowledge of white matter anatomy and to make an effort to apply this knowledge in routine reporting. INTRODUCTION (Fig. 1). However, the use of specific DTI sequences provides far more detailed and clini- DTI has allowed in vivo demonstration of axonal cally useful information. architecture and connectivity. This technique has set the stage for numerous studies on normal and abnormal connectivity and their role in devel- DIFFUSION TENSOR IMAGING: THE BASICS opmental and acquired disorders. Referencing established white matter anatomy, DTI atlases, Using appropriate magnetic field gradients, and neuroanatomical descriptions, this article diffusion-weighted sequences can be used to summarizes the major white matter anatomy and detect the motion of the water molecules to and related structures relevant to the clinical neurora- from cells. This free movement of the water mole- diologist in daily practice. -

Mesencephalon; Midbrain Mesencephalon; Midbrain
DOI: 10.5772/intechopen.68767 Provisional chapter Chapter 8 Mesencephalon; Midbrain Mesencephalon; Midbrain Ayla Kurkcuoglu Ayla Kurkcuoglu Additional information is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.68767 Abstract The mesencephalon is the most rostral part of the brainstem and sits above the pons and is adjoined rostrally to the thalamus. It comprises two lateral halves, called the cerebral peduncles; which is again divided into an anterior part, the crus cerebri, and a posterior part, tegmentum. The tectum is lay dorsal to an oblique coronal plane which includes the aquaduct, and consist of pretectal area and the corpora quadrigemina. In transvers section, the cerebral peduncles are seen to be composed of dorsal and ventral regions separated by the substantia nigra. Tegmentum mesencephali contains red nucleus, ocu‐ lomotor nucleus, thochlear nucleus, reticular nuclei, medial lemnisci, lateral lemnisci and medial longitudinal fasciculus. In tectum, the inferior colliculus and superior col‐ liculus have main nucleus, which are continuous with the periaqueductal grey matter. The mesencephalon serves important functions in motor movement, particularly move‐ ments of the eye, and in auditory and visual processing. The mesencephalic syndrome cause tremor, spastic paresis or paralysis, opisthotonos, nystagmus and depression or coma. In addition cranial trauma, brain tumors, thiamin deficiency and inflammatory or degenerative disorders of the mesencephalon have also been associated with the mid‐ brain syndrome. Keywords: the midbrain, mesencephalon, crus cerebri, substantia nigra, tectum 1. Introduction The nervous system has two components, namely the central nervous system and the periph‐ eral nervous system. -
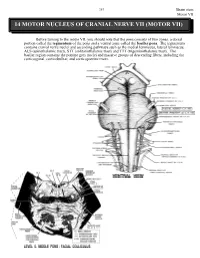
14 Motor Nucleus of Cranial Nerve Vii (Motor Vii)
263 Brain stem Motor VII 14 MOTOR NUCLEUS OF CRANIAL NERVE VII (MOTOR VII) Before turning to the motor VII, you should note that the pons consists of two zones, a dorsal portion called the tegmentum of the pons and a ventral zone called the basilar pons. The tegmentum contains cranial nerve nuclei and ascending pathways such as the medial lemniscus, lateral lemniscus, ALS (spinothalamic tract), STT (solitariothalamic tract) and TTT (trigeminothalamic tract). The basilar region contains the pontine grey nuclei and massive groups of descending fibers, including the corticospinal, corticobulbar, and corticopontine tracts. Brain stem 264 Motor VII The motor nucleus VII contains motor neurons (branchiomotor) that innervate the muscles of facial expression including the orbicularis oculi (CLOSES eyelid), the stapedius, the stylohyoid and the posterior belly of the digastric. Neurons comprising motor VII possess axons that pursue a rather circuitous route in order to exit the brain stem. Initially they pass dorsally and medially to loop over the abducens nucleus. The fibers then course ventrally and laterally to exit the brain stem. The bump in the floor of the fourth ventricle caused by the motor fibers of C.N. VII looping over the abducens nucleus is called the FACIAL COLLICULUS. A unilateral lesion interrupting the axons of C.N. VII results in the following: On the ipsilateral side, the forehead is immobile, the corner of the mouth sags, the nasolabial folds of the face are flattened, facial lines are lost, and saliva may drip from the corner of the mouth. The patient is unable to whistle or puff the cheek because the buccinator muscle is paralyzed. -

Destruction of the "Pyramidal Tract" in Man*
Destruction of the "Pyramidal Tract" in Man* PAUL C. BucY, M.D., JAMES E. KEPLINGER, M.D., AND EDIR B. SIQUEIRA,M.D. Department of Surgery, Northwestern University Medical School and Section on Neurological Surgery, Chicago Wesley Memorial Hospital, Chicago, Illinois PPORTUNITIES to observe the results cal "pyramidal tract") occupy the central of the isolated destruction of the portion of the cerebral peduncle (Fig. 1) as O "pyramidal tract" in man are rare. Levin ls,19 has shown, while the corticospinal There are but few places in the nervous sys- fibers from the postcentral region lie immedi- tem where the corticospinal fibers are sepa- ately lateral to these (Fig. ~).1 In the most rated sufficiently from other systems to per- medial part of the peduncle are found the mit of their isolated destruction, either surgi- frontopontine fibers, ls,19 The exact nature of cally or by disease. Extirpation of the the fibers in the most lateral part of the cere- pre- and postcentral cerebral cortex results in bral peduncle is less well known. Marin a destruction of the "pyramidal tract" but et al., 2~ from a study of human material con- also destroys other fibers, both ascending and cluded that the lateral segment of the pe- descending, thus producing a complex pic- duncle is composed of corticopontine fibers ture. Lesions of the internal capsule fre- from the parietal, occipital and temporal quently destroy the corticospinal system but lobes. However, their evidence as to occipito- they also destroy descending pathways to the pontine fibers is not conclusive, and that basal ganglia, the thalamus, the brain stem, dealing with a temporopontine component the cerebellum, etc., as well as many ascend- indicates only a very few such fibers.