Diffusion Tensor Tractography of Brainstem Fibers and Its
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Activation of Raphe Nuclei Triggers Rapid and Distinct Effects on Parallel Olfactory Bulb Output Channels
Activation of raphe nuclei triggers rapid and distinct effects on parallel olfactory bulb output channels The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Kapoor, Vikrant, Allison Provost, Prateek Agarwal, and Venkatesh N. Murthy. 2015. “Activation of raphe nuclei triggers rapid and distinct effects on parallel olfactory bulb output channels.” Nature neuroscience 19 (2): 271-282. doi:10.1038/nn.4219. http:// dx.doi.org/10.1038/nn.4219. Published Version doi:10.1038/nn.4219 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:29002684 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Nat Neurosci Manuscript Author . Author manuscript; Manuscript Author available in PMC 2016 August 01. Published in final edited form as: Nat Neurosci. 2016 February ; 19(2): 271–282. doi:10.1038/nn.4219. Activation of raphe nuclei triggers rapid and distinct effects on parallel olfactory bulb output channels Vikrant Kapoor1,2,†, Allison Provost1,2, Prateek Agarwal1,2, and Venkatesh N. Murthy1,2,† 1Center for Brain Science, Harvard University, Cambridge, MA 02138 2Department of Molecular & Cellular Biology, Harvard University, Cambridge, MA 02138 Abstract The serotonergic raphe nuclei are involved in regulating brain states over time-scales of minutes and hours. We examined more rapid effects of serotonergic activation on two classes of principal neurons in the mouse olfactory bulb, mitral and tufted cells, which send olfactory information to distinct targets. -

Characterization of Age-Related Microstructural Changes in Locus Coeruleus and Substantia Nigra Pars Compacta
UC Riverside UC Riverside Previously Published Works Title Characterization of age-related microstructural changes in locus coeruleus and substantia nigra pars compacta. Permalink https://escholarship.org/uc/item/08304755 Journal Neurobiology of aging, 87 ISSN 0197-4580 Authors Langley, Jason Hussain, Sana Flores, Justino J et al. Publication Date 2020-03-01 DOI 10.1016/j.neurobiolaging.2019.11.016 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Neurobiology of Aging xxx (2019) 1e9 Contents lists available at ScienceDirect Neurobiology of Aging journal homepage: www.elsevier.com/locate/neuaging Characterization of age-related microstructural changes in locus coeruleus and substantia nigra pars compacta Jason Langley a, Sana Hussain b, Justino J. Flores c, Ilana J. Bennett c, Xiaoping Hu a,b,* a Center for Advanced Neuroimaging, University of California Riverside, Riverside, CA, USA b Department of Bioengineering, University of California Riverside, Riverside, CA, USA c Department of Psychology, University of California Riverside, Riverside, CA, USA article info abstract Article history: Locus coeruleus (LC) and substantia nigra pars compacta (SNpc) degrade with normal aging, but not Received 26 May 2019 much is known regarding how these changes manifest in MRI images, or whether these markers predict Received in revised form 19 November 2019 aspects of cognition. Here, we use high-resolution diffusion-weighted MRI to investigate microstructural Accepted 22 November 2019 and compositional changes in LC and SNpc in young and older adult cohorts, as well as their relationship with cognition. In LC, the older cohort exhibited a significant reduction in mean and radial diffusivity, but a significant increase in fractional anisotropy compared with the young cohort. -

Scaling Our World View: How Monoamines Can Put Context Into Brain Circuitry
HYPOTHESIS AND THEORY published: 20 December 2018 doi: 10.3389/fncel.2018.00506 Scaling Our World View: How Monoamines Can Put Context Into Brain Circuitry Philipp Stratmann 1,2*, Alin Albu-Schäffer 1,2 and Henrik Jörntell 3 1 Sensor Based Robotic Systems and Intelligent Assistance Systems, Department of Informatics, Technical University of Munich, Garching, Germany, 2 German Aerospace Center (DLR), Institute of Robotics and Mechatronics, Weßling, Germany, 3 Neural Basis of Sensorimotor Control, Department of Experimental Medical Science, Lund University, Lund, Sweden Monoamines are presumed to be diffuse metabotropic neuromodulators of the topographically and temporally precise ionotropic circuitry which dominates CNS functions. Their malfunction is strongly implicated in motor and cognitive disorders, but their function in behavioral and cognitive processing is scarcely understood. In this paper, the principles of such a monoaminergic function are conceptualized for locomotor control. We find that the serotonergic system in the ventral spinal cord scales ionotropic signals and shows topographic order that agrees with differential gain modulation of ionotropic subcircuits. Whereas the subcircuits can collectively signal predictive models of the world based on life-long learning, their differential scaling continuously adjusts these models to changing mechanical contexts based on sensory input on a fast time scale of a few 100 ms. The control theory of biomimetic robots demonstrates that this precision scaling is an effective and resource-efficient -
Brainstem: Midbrainmidbrain
Brainstem:Brainstem: MidbrainMidbrain 1.1. MidbrainMidbrain –– grossgross externalexternal anatomyanatomy 2.2. InternalInternal structurestructure ofof thethe midbrain:midbrain: cerebral peduncles tegmentum tectum (guadrigeminal plate) Midbrain MidbrainMidbrain –– generalgeneral featuresfeatures location – between forebrain and hindbrain the smallest region of the brainstem – 6-7g the shortest brainstem segment ~ 2 cm long least differentiated brainstem division human midbrain is archipallian – shared general architecture with the most ancient of vertebrates embryonic origin – mesencephalon main functions:functions a sort of relay station for sound and visual information serves as a nerve pathway of the cerebral hemispheres controls the eye movement involved in control of body movement Prof. Dr. Nikolai Lazarov 2 Midbrain MidbrainMidbrain –– grossgross anatomyanatomy dorsal part – tectum (quadrigeminal plate): superior colliculi inferior colliculi cerebral aqueduct ventral part – cerebral peduncles:peduncles dorsal – tegmentum (central part) ventral – cerebral crus substantia nigra Prof. Dr. Nikolai Lazarov 3 Midbrain CerebralCerebral cruscrus –– internalinternal structurestructure CerebralCerebral peduncle:peduncle: crus cerebri tegmentum mesencephali substantia nigra two thick semilunar white matter bundles composition – somatotopically arranged motor tracts: corticospinal } pyramidal tracts – medial ⅔ corticobulbar corticopontine fibers: frontopontine tracts – medially temporopontine tracts – laterally -

Sex Differences in the Hypothalamic Oxytocin Pathway to Locus Coeruleus and Augmented Attention with Chemogenetic Activation of Hypothalamic Oxytocin Neurons
International Journal of Molecular Sciences Article Sex Differences in the Hypothalamic Oxytocin Pathway to Locus Coeruleus and Augmented Attention with Chemogenetic Activation of Hypothalamic Oxytocin Neurons Xin Wang, Joan B. Escobar and David Mendelowitz * Department of Pharmacology and Physiology, School of Medicine and Health Sciences, The George University Washington, Washington, DC 20037, USA; [email protected] (X.W.); [email protected] (J.B.E.) * Correspondence: [email protected] Abstract: The tightly localized noradrenergic neurons (NA) in the locus coeruleus (LC) are well recognized as essential for focused arousal and novelty-oriented responses, while many children with autism spectrum disorder (ASD) exhibit diminished attention, engagement and orienting to exogenous stimuli. This has led to the hypothesis that atypical LC activity may be involved in ASD. Oxytocin (OXT) neurons and receptors are known to play an important role in social behavior, pair bonding and cognitive processes and are under investigation as a potential treatment for ASD. However, little is known about the neurotransmission from hypothalamic paraventricular (PVN) OXT neurons to LC NA neurons. In this study, we test, in male and female rats, whether PVN OXT neurons excite LC neurons, whether oxytocin is released and involved in this neurotransmission, Citation: Wang, X.; Escobar, J.B.; and whether activation of PVN OXT neurons alters novel object recognition. Using “oxytocin sniffer Mendelowitz, D. Sex Differences in cells” (CHO cells that express the human oxytocin receptor and a Ca indicator) we show that there is the Hypothalamic Oxytocin Pathway release of OXT from hypothalamic PVN OXT fibers in the LC. Optogenetic excitation of PVN OXT to Locus Coeruleus and Augmented fibers excites LC NA neurons by co-release of OXT and glutamate, and this neurotransmission is Attention with Chemogenetic Activation of Hypothalamic Oxytocin greater in males than females. -

Corticotropin-Releasing Factor in the Norepinephrine Nucleus, Locus Coeruleus, Facilitates Behavioral Flexibility
Neuropsychopharmacology (2012) 37, 520–530 & 2012 American College of Neuropsychopharmacology. All rights reserved 0893-133X/12 www.neuropsychopharmacology.org Corticotropin-Releasing Factor in the Norepinephrine Nucleus, Locus Coeruleus, Facilitates Behavioral Flexibility Kevin Snyder1,4, Wei-Wen Wang2,4, Rebecca Han1, Kile McFadden3 and Rita J Valentino*,3 1 2 3 The University of Pennsylvania, Philadelphia, PA, USA; Key Laboratory of Mental Health, Institute of Psychology, Beijing, China; Department of Anesthesiology and Critical Care Medicine, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA Corticotropin-releasing factor (CRF), the stress-related neuropeptide, acts as a neurotransmitter in the brain norepinephrine nucleus, locus coeruleus (LC), to activate this system during stress. CRF shifts the mode of LC discharge from a phasic to a high tonic state that is thought to promote behavioral flexibility. To investigate this, the effects of CRF administered either intracerebroventricularly (30–300 ng, i.c.v.) or directly into the LC (intra-LC; 2–20 ng) were examined in a rat model of attentional set shifting. CRF differentially affected components of the task depending on dose and route of administration. Intracerebroventricular CRF impaired intradimensional set shifting, reversal learning, and extradimensional set shifting (EDS) at different doses. In contrast, intra-LC CRF did not impair any aspect of the task. The highest dose of CRF (20 ng) facilitated reversal learning and the lowest dose (2 ng) improved EDS. The dose–response relationship for CRF on EDS performance resembled an inverted U-shaped curve with the highest dose having no effect. Intra-LC CRF also elicited c-fos expression in prefrontal cortical neurons with an inverted U-shaped dose–response relationship. -

Overview of the Reticular Formation (RF)
TeachSheet Reticular Formation & Diffuse modulatory system Overview of the Reticular Formation (RF) The term reticular formation refers to the neuronal network within the brainstem, although it continues rostrally into the thalamus and hypothalamus and caudally into the propriospinal network of the spinal cord. A “coordinating system” (like the Limbic system) with “connections” to sensory, somatic motor and visceral motor systems Organization can be subdivided into two neuronal cell “columns” (medial to lateral) as well as on the basis of their neurotransmitter release Neuronal columns (many nuclei and names, but these are some of the major ones): o “Medial tegmental field” (large-celled nuclei) . Origin of the reticulospinal pathway . Role in coordinating posture, eye and head movements. o “Lateral tegmental field” (smaller and fewer cells, shorter local projections) . Extends from the medulla to the pons . Coordinate autonomic and limbic functions (e.g. micturition, swallowing, mastication and vocalization) The functions of the reticular formation include their ability to coordinate motor and sensory brainstem nuclei: o Pattern generator . Eye movements; horizontal (PPRF) and vertical (riMLF) . Rhythmical chewing movements (pons) . Posture and locomotion (midbrain and pons) . Swallowing, vomiting, coughing and sneezing (medulla) . Micturition (pons) o Respiratory control (medulla); expiratory, inspiratory, apneustic and pneumotaxic o Cardiovascular control (medulla); vasomotor pressor/depressor, cardioacceleratory and inhibitory. Afferents arise from baroreceptors (carotid sinus and aortic arch), chemoreceptors (carotid sinus, lateral reticular formation chemosensitive area in the medulla) and stretch receptors (lung and respiratory muscles) . Efferents arise from reticular formation neurons within the pons and medulla o Sensory modulation or “gate” control . The term “gating” refers to “modulation” of synaptic transmission from one set of neurons to the next. -
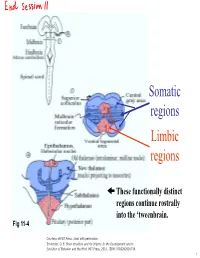
Lecture 12 Notes
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -

Neuromelanin Marks the Spot: Identifying a Locus Coeruleus Biomarker of Cognitive Reserve in Healthy Aging
Neurobiology of Aging xxx (2015) 1e10 Contents lists available at ScienceDirect Neurobiology of Aging journal homepage: www.elsevier.com/locate/neuaging Neuromelanin marks the spot: identifying a locus coeruleus biomarker of cognitive reserve in healthy aging David V. Clewett a,*, Tae-Ho Lee b, Steven Greening b,c,d, Allison Ponzio c, Eshed Margalit e, Mara Mather a,b,c a Neuroscience Graduate Program, University of Southern California, Los Angeles, CA, USA b Department of Psychology, University of Southern California, Los Angeles, CA, USA c Davis School of Gerontology, University of Southern California, Los Angeles, CA, USA d Department of Psychology, Louisiana State University, Baton Rouge, LA, USA e Dornsife College of Letters and Sciences, University of Southern California, Los Angeles, CA, USA article info abstract Article history: Leading a mentally stimulating life may build up a reserve of neural and mental resources that preserve Received 28 May 2015 cognitive abilities in late life. Recent autopsy evidence links neuronal density in the locus coeruleus (LC), Received in revised form 18 September 2015 the brain’s main source of norepinephrine, to slower cognitive decline before death, inspiring the idea Accepted 23 September 2015 that the noradrenergic system is a key component of reserve (Robertson, I. H. 2013. A noradrenergic theory of cognitive reserve: implications for Alzheimer’s disease. Neurobiol. Aging. 34, 298e308). Here, we tested this hypothesis using neuromelanin-sensitive magnetic resonance imaging to visualize and Keywords: measure LC signal intensity in healthy younger and older adults. Established proxies of reserve, including Locus coeruleus Aging education, occupational attainment, and verbal intelligence, were linearly correlated with LC signal in- fi Norepinephrine tensity in both age groups. -

Brain Structure and Function Related to Headache
Review Cephalalgia 0(0) 1–26 ! International Headache Society 2018 Brain structure and function related Reprints and permissions: sagepub.co.uk/journalsPermissions.nav to headache: Brainstem structure and DOI: 10.1177/0333102418784698 function in headache journals.sagepub.com/home/cep Marta Vila-Pueyo1 , Jan Hoffmann2 , Marcela Romero-Reyes3 and Simon Akerman3 Abstract Objective: To review and discuss the literature relevant to the role of brainstem structure and function in headache. Background: Primary headache disorders, such as migraine and cluster headache, are considered disorders of the brain. As well as head-related pain, these headache disorders are also associated with other neurological symptoms, such as those related to sensory, homeostatic, autonomic, cognitive and affective processing that can all occur before, during or even after headache has ceased. Many imaging studies demonstrate activation in brainstem areas that appear specifically associated with headache disorders, especially migraine, which may be related to the mechanisms of many of these symptoms. This is further supported by preclinical studies, which demonstrate that modulation of specific brainstem nuclei alters sensory processing relevant to these symptoms, including headache, cranial autonomic responses and homeostatic mechanisms. Review focus: This review will specifically focus on the role of brainstem structures relevant to primary headaches, including medullary, pontine, and midbrain, and describe their functional role and how they relate to mechanisms -

Distribution of Serotonin Receptors and Transporters in the Human Brain: Implications for Psychosis
From the Department of Clinical Neuroscience Karolinska Institutet, Stockholm, Sweden Distribution of Serotonin Receptors and Transporters in the Human Brain: Implications for Psychosis Katarina Varnäs Stockholm 2005 © Katarina Varnäs, 2005 ISBN 91-7140-280-2 Though this be madness, yet there is method in it. William Shakespeare ABSTRACT The serotonin (5-HT) system is thought to be involved in different psychiatric disorders, includ- ing depression and anxiety disorders, and is a major target for the pharmacological treatment of these conditions. The involvement of the 5-HT system in psychosis has been suggested, as 5- HT receptor agonism is a common mechanism of different classes of hallucinogenic drugs and several antipsychotics are 5-HT receptor antagonists. In this study, the distribution of 5-HT transporters and G-protein-coupled 5-HT recep- tors in the human brain was characterized using whole hemisphere autoradiographic tech- niques. In addition, a pilot investigation was performed to compare the densities of 5-HT bind- ing sites in brain tissue from patients who suffered from schizophrenia-like psychosis and con- trol subjects. We found higher levels of 5-HT transporters in several regions of the greater limbic lobe (subcallosal area, anterior cingulate gyrus, posterior uncus, insular and entorhinal cortices, and the temporal pole) as compared to the isocortex. Higher levels of 5-HT 1A receptors were also found in limbic cortices (subcallosal area, temporal pole, hippocampus, and entorhinal cortex) compared to isocortical structures. Dense binding to 5-HT 1B receptors was found in the ventral striato-pallidal system of the human brain. 5-HT 1B receptor mRNA expression was detected in the striatum with the highest levels in ventral striatal regions. -

Neuroanatomy
Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Neuroanatomy W. Jeffrey Wilson Fall 2012 \Without education we are in a horrible and deadly danger of taking educated people seriously." { Gilbert Keith Chesterton [LATEX in use { a Microsoft- & PowerPoint-free presentation] Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Blood-Brain Barrier Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Peripheral Nervous System • Somatic N.S.: skeletal muscles, skin, joints • Autonomic N.S.: internal organs, glands • Sympathetic N.S.: rapid expenditure of energy • Parasympathetic N.S.: restoration of energy Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Spinal Cord Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain | Ventricles Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Hindbrain Myelencephalon & Metencephalon Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Reticular