(Orthoptera: Tettigoniidae) Species Composition and Size Across Latitude in Atlantic Coast Salt Marshes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

THE QUARTERLY REVIEW of BIOLOGY
VOL. 43, NO. I March, 1968 THE QUARTERLY REVIEW of BIOLOGY LIFE CYCLE ORIGINS, SPECIATION, AND RELATED PHENOMENA IN CRICKETS BY RICHARD D. ALEXANDER Museum of Zoology and Departmentof Zoology The Universityof Michigan,Ann Arbor ABSTRACT Seven general kinds of life cycles are known among crickets; they differ chieff,y in overwintering (diapause) stage and number of generations per season, or diapauses per generation. Some species with broad north-south ranges vary in these respects, spanning wholly or in part certain of the gaps between cycles and suggesting how some of the differences originated. Species with a particular cycle have predictable responses to photoperiod and temperature regimes that affect behavior, development time, wing length, bod)• size, and other characteristics. Some polymorphic tendencies also correlate with habitat permanence, and some are influenced by population density. Genera and subfamilies with several kinds of life cycles usually have proportionately more species in temperate regions than those with but one or two cycles, although numbers of species in all widely distributed groups diminish toward the higher lati tudes. The tendency of various field cricket species to become double-cycled at certain latitudes appears to have resulted in speciation without geographic isolation in at least one case. Intermediate steps in this allochronic speciation process are illustrated by North American and Japanese species; the possibility that this process has also occurred in other kinds of temperate insects is discussed. INTRODUCTION the Gryllidae at least to the Jurassic Period (Zeuner, 1939), and many of the larger sub RICKETS are insects of the Family families and genera have spread across two Gryllidae in the Order Orthoptera, or more continents. -

Prairie Fen and Associated Savanna Restoration Submittal Package
Region 3 Wildlife and Sport Fish Restoration Transmittal Form U.S. Fish and Wildlife Service WSFR Bishop Henry Whipple Federal Building One Federal Drive Fort Snelling, MN 55111-4056 Date Received: Date Approval Requested by: Friday, September 25, 2009 (Region 3 FA Date Stamp) Date Submitted: Thursday, August 27, 2009 Project No.: To Be Determined Project Title: Prairie Fen and Associated Savanna Restoration in Michigan and Indiana for Species of Greatest Conservation Need State Contact: Eric Sink FWS Biologist: Mike Sweet, (612) 713-5129 Telephone Number: (517) 335-1064 e-mail address: [email protected] Track (check one): Routine = 15 day Non-Routine = 30 day Non-Routine = 45 day Type(s): Grant Proposal (GP): GP Renewal: Grant Segment: GP Amend: Segment Amend: USFWS will complete USFWS will complete Obligation Intent: Sub-Account Federal Share Circle or Check if Applicable: WR Regular 5220 $ In-Kind Value Program Income WR Sect 4 Hunter Ed 5210 $ SHPO WR Sect 10 Hunter Ed 523 $ NEPA (EA Letter or EA) Section 7 WL Cons. & Rest. 5511 $ Lobby Certification SFR Regular 9514 $ Other (describe in Note/Special Instructions section below) SFR Aquatic Ed. 9511 $ Needs funding condition (describe in Note/Special Instructions section below) SFR Boat Access 9521 $ COMPETITIVE ( ) or NON-COMPETITIVE ( ) Other : $ 864,020 Competitive SWG Notes/Special Instructions (check): Biologist Fiscal Land Secretary Mike, until we can get the Section 7 consultation and biological opinion documents, can you approve with the caveate that no work is to be done -

The Taxonomy of Utah Orthoptera
Great Basin Naturalist Volume 14 Number 3 – Number 4 Article 1 12-30-1954 The taxonomy of Utah Orthoptera Andrew H. Barnum Brigham Young University Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation Barnum, Andrew H. (1954) "The taxonomy of Utah Orthoptera," Great Basin Naturalist: Vol. 14 : No. 3 , Article 1. Available at: https://scholarsarchive.byu.edu/gbn/vol14/iss3/1 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. IMUS.COMP.ZSOL iU6 1 195^ The Great Basin Naturalist harvard Published by the HWIilIijM i Department of Zoology and Entomology Brigham Young University, Provo, Utah Volum e XIV DECEMBER 30, 1954 Nos. 3 & 4 THE TAXONOMY OF UTAH ORTHOPTERA^ ANDREW H. BARNUM- Grand Junction, Colorado INTRODUCTION During the years of 1950 to 1952 a study of the taxonomy and distribution of the Utah Orthoptera was made at the Brigham Young University by the author under the direction of Dr. Vasco M. Tan- ner. This resulted in a listing of the species found in the State. Taxonomic keys were made and compiled covering these species. Distributional notes where available were made with the brief des- criptions of the species. The work was based on the material in the entomological col- lection of the Brigham Young University, with additional records obtained from the collection of the Utah State Agricultural College. -

The Evolution of Complex Calls in Meadow
THE EVOLUTION OF COMPLEX CALLS IN MEADOW KATYDIDS _______________________________________ A Dissertation presented to the Faculty of the Graduate School at the University of Missouri-Columbia _______________________________________________________ In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy _____________________________________________________ by NATHAN HARNESS Dr. Johannes Schul, Dissertation Supervisor July 2018 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled THE EVOLUTION OF COMPLEX CALLS IN MEADOW KATYDIDS presented by Nathan Harness, a candidate for the degree of doctor of philosophy, and hereby certify that, in their opinion, it is worthy of acceptance. Professor Johannes Schul Professor Sarah Bush Professor Lori Eggert Professor Patricia Friedrichsen For my family Rachel and Mayr have given me so much. They show me unselfish affection, endless support, and generosity that seems to only grow. Without them the work here, and the adventure we’ve all three gone on surrounding it, would not have been possible. They have sacrificed birthdays, anniversaries, holidays, and countless weekends and evenings. They’ve happily seen me off to weeks of field work and conference visits. I am thankful to them for being so generous, and completely lacking in resentment at all the things that pull their husband and dad in so many directions. They have both necessarily become adept at melting away anxiety; I will forever be indebted to the hugs of a two-year-old and the kind words of his mom. Rachel and Mayr both deserve far more recognition than is possible here. I also want to thank my parents and brother and sisters. -

Lepidoptera: Noctuidae
Life: The Excitement of Biology 5(2) 115 Orchelimum gladiator Bruner, 1891: An Addition to the Prey Record of the Great Black Wasp, Sphex pensylvanicus Linnaeus, 1763 (Hymenoptera: Sphecidae: Sphecinae)1 G. K. Lechner2 The Great Black Wasp, Sphex pensylvanicus Linnaeus, 1763 is a known predator of katydids (Orthoptera: Tettigoniidae) and is rather catholic in its choice of prey seemingly taking any katydid species available. In a previous paper (Lechner 2016), I stated that five species placed in four genera of katydids have been reported as prey items of S. pensylvanicus. Also, I reported four confirmed katydid species that I took from S. pensylvanicus that nest in the soil behind a vertical retaining wall on my residential property in Sioux City, Iowa. Herein, I add the Gladiator Meadow Katydid, Orchelimum gladiator Bruner, 1891 (Figures 1 and 2, next page), to the prey record of S. pensylvanicus. I took this paralyzed katydid from its captor female S. pensylvanicus on 28 August 2017. At that time, the wasp was attempting to enter her nest with her prey item in the north lot line retaining wall. Acknowledgments Many thanks to Dr. Thomas J. Walker (University of Florida, Gainesville, Florida, USA) for identifying the specimen of Orchcelimum gladiator. This katydid was donated to the Ensiferan Collection at the University of Florida. Literature Cited Lechner, G. K. 2016. Interesting incidents with Sphex pensylvanicus Linnaeus, 1763 (Hymenoptera: Sphecidae) wasps and their prey items in Sioux City, Iowa, U.S.A. Life: The Excitement of Biology 4:27-31. https://blaypublishers.files.wordpress.com/2016/07/lechner-leb-41-interesting- incidents-with-sphex-pensylvanicus-linnaeus.pdf 1 Received on October 17, 2017. -

Preliminary Checklist of the Orthopteroid Insects (Blattodea, Mantodea, Phasmatodea,Orthoptera) of Texas
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida March 2001 Preliminary checklist of the orthopteroid insects (Blattodea, Mantodea, Phasmatodea,Orthoptera) of Texas John A. Stidham Garland, TX Thomas A. Stidham University of California, Berkeley, CA Follow this and additional works at: https://digitalcommons.unl.edu/insectamundi Part of the Entomology Commons Stidham, John A. and Stidham, Thomas A., "Preliminary checklist of the orthopteroid insects (Blattodea, Mantodea, Phasmatodea,Orthoptera) of Texas" (2001). Insecta Mundi. 180. https://digitalcommons.unl.edu/insectamundi/180 This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. INSECTA MUNDI, Vol. 15, No. 1, March, 2001 35 Preliminary checklist of the orthopteroid insects (Blattodea, Mantodea, Phasmatodea,Orthoptera) of Texas John A. Stidham 301 Pebble Creek Dr., Garland, TX 75040 and Thomas A. Stidham Department of Integrative Biology, Museum of Paleontology, and Museum of Vertebrate Zoology, University of California, Berkeley, CA 94720, Abstract: Texas has one of the most diverse orthopteroid assemblages of any state in the United States, reflecting the varied habitats found in the state. Three hundred and eighty-nine species and 78 subspecies of orthopteroid insects (Blattodea, Mantodea, Phasmatodea, and Orthoptera) have published records for the state of Texas. This is the first such comprehensive checklist for Texas and should aid future work on these groups in this area. Introduction (Flook and Rowell, 1997). -

Great Lakes Entomologist
The GREAT LAKES ENTOMOLOGIST Vol. 5, No. 2 Summer 1972 The Singing Insects of Michigan RichardD. Alexander, Ann E. Pace and Daniel Otte THE GREAT LAKES ENTOMOLOGIST Published by the Michigan Entomological Society Volume 5 1972 No. 2 TABLE OF CONTENTS The singing insects of Michigan Richard D. Alexander, Ann E. Pace and Daniel Otte . .33 COVER ILLUSTRATION The Northern True Katydid, Pterophylla camellifolia (Fabricius) (Orthoptera: Tet- tigoniidae), whose raucus calls of "katydid, katy-did" can be heard from the tops of deciduous trees in the southern part of the Lower Peninsula during the evenings of middle and late summer. THE MICHIGAN ENTOMOLOGICAL SOCIETY 197 1-1972 OFFICERS President Dean G. DiIlery President-Elect Richard C. Fleming Executive Secretary M. C. Nielsen Editor Irving J. Cantrall The Michigan Entomological Society traces its origins'to the old Detroit Entomological Society and was organized on 4 November 1954 to ". promote the science of entomology in all its branches and by all feasible means, and to advance cooperation and good fellowship among persons interested in entomology." The Society attempts to facilitate the exchange of ideas and information in both amateur and professional circles, and encourages the study of insects by youth. Membership in the Society, which serves the North Central States and adjacent Canada, is open to all persons interested in entomology. There are three paying classes of membership: Student (including those currently enrolled in college or graduate programs) - annual dues $2.00 Active - anriual dues $4.00 Institutional - annual dues $6.00 Sustaining - annual contribution $25.00 or more Dues are paid on a calendar year basis (Jan. -

Orthoptera: Tettigoniidae: Conocephalinae: Copiphorini)
THOMAS J. WALKERJournal of Orthoptera Research 2014, 23(1): 69-7369 A new North American species of Bucrates (Orthoptera: Tettigoniidae: Conocephalinae: Copiphorini) THOMAS J. WALKER Department of Entomology & Nematology, University of Florida, Gainesville, FL 32611, USA. Email: [email protected] Abstract Methods Bucrates weissmani n.sp. is known from four localities in southern Access to supporting materials (SM).—To improve access to the origi- Arizona. It is smaller and more slender than the other four species of nal data and the analyses that were undertaken for this paper, four Bucrates, making it superficially similar to the Central American copiphorine tables were composed and assigned to SM. These may be accessed Caulopsis cuspidata, but more fundamental features refute the notion that through hyperlinks in BioOne's "Full Text" and PDF digital versions. it belongs in Caulopsis rather than Bucrates. Four other species of Bucrates are known. Two of these, capitatus (De Geer) and clausus (Scudder), occur in sympatry in Central America and tropical South America; lanista Rehn is Collecting sites of specimens examined.—D.B. Weissman and Jeff Cole known only from southern Brazil; and malivolans (Scudder) is restricted to provided specimens of the new species from the five sites listed the southeastern United States. All are easily distinguished morphologically below. Unless within brackets, [.], the data are from labels on the and, for the three for which the songs are known, by their songs. Unlike the specimens. two other species of Bucrates for which the habitat is known, B. weissmani occurs on altitudinal islands at the edge of a desert. The calling song of B. -

New Canadian and Ontario Orthopteroid Records, and an Updated Checklist of the Orthoptera of Ontario
Checklist of Ontario Orthoptera (cont.) JESO Volume 145, 2014 NEW CANADIAN AND ONTARIO ORTHOPTEROID RECORDS, AND AN UPDATED CHECKLIST OF THE ORTHOPTERA OF ONTARIO S. M. PAIERO1* AND S. A. MARSHALL1 1School of Environmental Sciences, University of Guelph, Guelph, Ontario, Canada N1G 2W1 email, [email protected] Abstract J. ent. Soc. Ont. 145: 61–76 The following seven orthopteroid taxa are recorded from Canada for the first time: Anaxipha species 1, Cyrtoxipha gundlachi Saussure, Chloroscirtus forcipatus (Brunner von Wattenwyl), Neoconocephalus exiliscanorus (Davis), Camptonotus carolinensis (Gerstaeker), Scapteriscus borellii Linnaeus, and Melanoplus punctulatus griseus (Thomas). One further species, Neoconocephalus retusus (Scudder) is recorded from Ontario for the first time. An updated checklist of the orthopteroids of Ontario is provided, along with notes on changes in nomenclature. Published December 2014 Introduction Vickery and Kevan (1985) and Vickery and Scudder (1987) reviewed and listed the orthopteroid species known from Canada and Alaska, including 141 species from Ontario. A further 15 species have been recorded from Ontario since then (Skevington et al. 2001, Marshall et al. 2004, Paiero et al. 2010) and we here add another eight species or subspecies, of which seven are also new Canadian records. Notes on several significant provincial range extensions also are given, including two species originally recorded from Ontario on bugguide.net. Voucher specimens examined here are deposited in the University of Guelph Insect Collection (DEBU), unless otherwise noted. New Canadian records Anaxipha species 1 (Figs 1, 2) (Gryllidae: Trigidoniinae) This species, similar in appearance to the Florida endemic Anaxipha calusa * Author to whom all correspondence should be addressed. -

Grasshopper,Katydid,Crickets of Eoa Species Common Name
GRASSHOPPER,KATYDID,CRICKETS OF EOA SPECIES COMMON NAME Allonemobius allardi Allards ground cricket Allonemobius fasciatus striped ground cricket Allonemobius maculatus spotted ground cricket Allonemobius tinnulus tinkling ground cricket Amblycorpha alexanderi clicker round-wing katydid Amblycorpha longinicta common virtuoso katydid Amblycorpha oblongifolia oblong-winged katydid Amblycorpha rotundifolia rattler round-winged katydid Arphia simplex Plains yellow-winged grasshopper Arphia sulphurea spring yellow-winged grasshopper Arphia xanthoptera autumn yellow-wing grasshopper Atlanticus americanus American sheildback Camptonotus carlinensis Carolina leaf roller Ceuthophilus brevipes short-legged camel cricket Ceuthophilus g. gracilipes camel cricket Ceuthophilus uhleri Uhler's camel cricket Chortophaga viridifasciata N. green-striped hopper Conocephalus attenuatus long-tailed meadow katydid Conocephalus brevipennis short-winged meadow katydid Conocephalus fasciatus slender meadow katydid Conocephalus nemoralis woodland meadow katydid Dicromorpha viridis short-wing green grasshopper Dissosteira carolina Carolina grasshopper Ellipes minutus minute pygmy mole grasshopper Encoptolophus soridus dusky grasshopper Gryllus pennsylvanicus fall field cricket Melanolplus obovatipennis obovate-winged grasshopper Melanoplus bivittatus two-striped grasshopper Melanoplus differentialis differential grasshopper Melanoplus femurrubrum red-legged grasshopper Melanoplus gracilis graceful grasshopper Melanoplus keeleri luridus Keelers grasshopper Melanoplus -
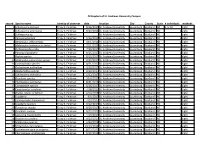
Orthoptera of St. Andrews University Campus Record Species Name
Orthoptera of St. Andrews University Campus record Species name Identity of observer date location City County State # individuals methods 1 Schistocerca obscura Tracy S. Feldman 8/16/2017 St. Andrews University Laurinburg Scotland NC 5 or 10 sight 2 Schistocerca americana Tracy S. Feldman 9/23/2014 St. Andrews University Laurinburg Scotland NC 5 sight 3 Schistocerca sp. Tracy S. Feldman St. Andrews University Laurinburg Scotland NC sight 4 Paroxya atlantica Tracy S. Feldman 9/16/2014 St. Andrews University Laurinburg Scotland NC 1 sight 5 Melanoplus differentialis Tracy S. Feldman 10/1/2014 St. Andrews University Laurinburg Scotland NC 1 sight 6 Melanoplus bivittatus or keeleri Tracy S. Feldman 7/21/2015 St. Andrews University Laurinburg Scotland NC 1 sight 7 Dichromorpha viridis Tracy S. Feldman 7/20/2017 St. Andrews University Laurinburg Scotland NC 1 sight 8 Paroxya clavuligera Tracy S. Feldman 8/15/2017 St. Andrews University Laurinburg Scotland NC 1 sight 9 Arphia species Tracy S. Feldman 8/15/2017 St. Andrews University Laurinburg Scotland NC 1 sight 10 Melanoplus sanguinipes group Tracy S. Feldman 9/20/2017 St. Andrews University Laurinburg Scotland NC 1 sight 11 Conocephalus species Tracy S. Feldman 8/21/2017 St. Andrews University Laurinburg Scotland NC 1 sight 12 Orchelimum pulchellum Tracy S. Feldman 9/20/2017 St. Andrews University Laurinburg Scotland NC 1 sight 13 Allonemobius species Tracy S. Feldman 9/20/2017 St. Andrews University Laurinburg Scotland NC 1 sight 14 Schistocerca damnifica Tracy S. Feldman 4/12/2017 St. Andrews University Laurinburg Scotland NC 1 sight 15 Orocharis species Tracy S. -

Natural Heritage Program List of Rare Animal Species of North Carolina 2020
Natural Heritage Program List of Rare Animal Species of North Carolina 2020 Hickory Nut Gorge Green Salamander (Aneides caryaensis) Photo by Austin Patton 2014 Compiled by Judith Ratcliffe, Zoologist North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Animal Species of North Carolina 2020 Compiled by Judith Ratcliffe, Zoologist North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. The list is published periodically, generally every two years.