Identification and Description of Mineral Processing Sectors and Waste Streams
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vanadium Pentoxide and Other Inorganic Vanadium Compounds
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization. Concise International Chemical Assessment Document 29 VANADIUM PENTOXIDE AND OTHER INORGANIC VANADIUM COMPOUNDS Note that the layout and pagination of this pdf file are not identical to the printed CICAD First draft prepared by Dr M. Costigan and Mr R. Cary, Health and Safety Executive, Liverpool, United Kingdom, and Dr S. Dobson, Centre for Ecology and Hydrology, Huntingdon, United Kingdom Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 2001 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management -

A Review of Flotation Separation of Mg Carbonates (Dolomite and Magnesite)
minerals Review A Review of Flotation Separation of Mg Carbonates (Dolomite and Magnesite) Darius G. Wonyen 1,†, Varney Kromah 1,†, Borbor Gibson 1,† ID , Solomon Nah 1,† and Saeed Chehreh Chelgani 1,2,* ID 1 Department of Geology and Mining Engineering, Faculty of Engineering, University of Liberia, P.O. Box 9020 Monrovia, Liberia; [email protected] (D.G.W.); [email protected] (Y.K.); [email protected] (B.G.); [email protected] (S.N.) 2 Department of Electrical Engineering and Computer Science, University of Michigan, Ann Arbor, MI 48109, USA * Correspondence: [email protected]; Tel.: +1-41-6830-9356 † These authors contributed equally to the study. Received: 24 July 2018; Accepted: 13 August 2018; Published: 15 August 2018 Abstract: It is well documented that flotation has high economic viability for the beneficiation of valuable minerals when their main ore bodies contain magnesium (Mg) carbonates such as dolomite and magnesite. Flotation separation of Mg carbonates from their associated valuable minerals (AVMs) presents several challenges, and Mg carbonates have high levels of adverse effects on separation efficiency. These complexities can be attributed to various reasons: Mg carbonates are naturally hydrophilic, soluble, and exhibit similar surface characteristics as their AVMs. This study presents a compilation of various parameters, including zeta potential, pH, particle size, reagents (collectors, depressant, and modifiers), and bio-flotation, which were examined in several investigations into separating Mg carbonates from their AVMs by froth flotation. Keywords: dolomite; magnesite; flotation; bio-flotation 1. Introduction Magnesium (Mg) carbonates (salt-type minerals) are typical gangue phases associated with several valuable minerals, and have complicated processing [1,2]. -

Calcination) 2
Thermal Treatment of Catalysts Modern Methods in Heterogeneous Catalysis Research Friederike C. Jentoft, October 31, 2003 Outline 1. Terminology (calcination) 2. Sample vs. oven set temperature 3. Self-generated atmosphere & self-steaming of zeolites 4. Combustion 5. Glow phenomenon & zirconia catalysts 6. Crystallization 7. Loss of surface area 8. Effect of additives 9. Solid-solid wetting 10.Reductive treatments & SMSI Steps of Catalyst Preparation v IUPAC defines 3 steps of catalyst preparation 1. Preparation of primary solid, associating all the useful compounds 2. Processing of that primary solid to obtain the catalyst precursor for example by heat treatment 3. Activation of the precursor to give the active catalyst (reduction to metal, formation of sulfides, deammoniation of zeolites) Heat Treatment of Intermediate Solids or Precursors v drying v thermal decomposition of salts (nitrates, ammonium salts) v calcination v product is a "reasonably inert solid" which can be stored easily Annealing v in a general meaning: a heating of a material over a long time span; strain and cracks in a crystalline solid can be removed Origin of the Term "Calcination" v latin "calx" = playstone limestone, (greek chálix) v burning of calcium carbonate (limestone) to calcium oxide (quicklime) -1 CaCO3 → CaO + CO2 ΔH(900°C)=3010 kJ mol v used to construct Giza pyramids (ca. 2800 A.C.), burning of limestone ("Kalkbrennen") mentioned by Cato 184 A.C. v performed in kilns (ovens) at 900°C v addition of air to sustain combustion + cool product Examples for -

Principles of Extractive Metallurgy Lectures Note
PRINCIPLES OF EXTRACTIVE METALLURGY B.TECH, 3RD SEMESTER LECTURES NOTE BY SAGAR NAYAK DR. KALI CHARAN SABAT DEPARTMENT OF METALLURGICAL AND MATERIALS ENGINEERING PARALA MAHARAJA ENGINEERING COLLEGE, BERHAMPUR DISCLAIMER This document does not claim any originality and cannot be used as a substitute for prescribed textbooks. The information presented here is merely a collection by the author for their respective teaching assignments as an additional tool for the teaching-learning process. Various sources as mentioned at the reference of the document as well as freely available material from internet were consulted for preparing this document. The ownership of the information lies with the respective author or institutions. Further, this document is not intended to be used for commercial purpose and the faculty is not accountable for any issues, legal or otherwise, arising out of use of this document. The committee faculty members make no representations or warranties with respect to the accuracy or completeness of the contents of this document and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. BPUT SYLLABUS PRINCIPLES OF EXTRACTIVE METALLURGY (3-1-0) MODULE I (14 HOURS) Unit processes in Pyro metallurgy: Calcination and roasting, sintering, smelting, converting, reduction, smelting-reduction, Metallothermic and hydrogen reduction; distillation and other physical and chemical refining methods: Fire refining, Zone refining, Liquation and Cupellation. Small problems related to pyro metallurgy. MODULE II (14 HOURS) Unit processes in Hydrometallurgy: Leaching practice: In situ leaching, Dump and heap leaching, Percolation leaching, Agitation leaching, Purification of leach liquor, Kinetics of Leaching; Bio- leaching: Recovery of metals from Leach liquor by Solvent Extraction, Ion exchange , Precipitation and Cementation process. -

Kinetic Studies of Sodium and Metforminium Decavanadates Decomposition and in Vitro Cytotoxicity and Insulin- Like Activity
inorganics Article Kinetic Studies of Sodium and Metforminium Decavanadates Decomposition and In Vitro Cytotoxicity and Insulin- Like Activity Aniela M. Silva-Nolasco 1,2, Luz Camacho 2 , Rafael Omar Saavedra-Díaz 1, Oswaldo Hernández-Abreu 1 , Ignacio E. León 3 and Irma Sánchez-Lombardo 1,* 1 Centro de Investigación de Ciencia y Tecnología Aplicada de Tabasco, División Académica de Ciencias Básicas (CICTAT), Universidad Juárez Autónoma de Tabasco, Carretera Cunduacán-Jalpa km. 1 Col. La Esmeralda, Cunduacán 86690, Tabasco, Mexico; [email protected] (A.M.S.-N.); [email protected] (R.O.S.-D.); [email protected] (O.H.-A.) 2 Laboratorio de Nutrición Experimental, Instituto Nacional de Pediatría, Ciudad de Mexico 04530, Mexico; [email protected] 3 Centro de Química Inorgánica CEQUINOR (CONICET, UNLP), Bv 120 1465, La Plata 1900, Argentina; [email protected] * Correspondence: [email protected] Received: 22 October 2020; Accepted: 2 December 2020; Published: 8 December 2020 Abstract: The kinetics of the decomposition of 0.5 and 1.0 mM sodium decavanadate (NaDeca) and metforminium decavanadate (MetfDeca) solutions were studied by 51V NMR in Dulbecco’s modified Eagle’s medium (DMEM) medium (pH 7.4) at 25 ◦C. The results showed that decomposition products 2 4 are orthovanadate [H2VO4]− (V1) and metavanadate species like [H2V2O7] − (V2), [V4O12] − (V4) 5 and [V5O15] − (V5) for both compounds. The calculated half-life times of the decomposition reaction were 9 and 11 h for NaDeca and MetfDeca, respectively, at 1 mM concentration. The hydrolysis products that presented the highest rate constants were V1 and V4 for both compounds. -
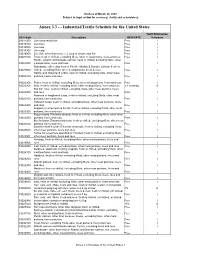
US Schedule for Internet V2
Draft as of March 23, 2007 Subject to legal review for accuracy, clarity and consistency. Annex 3.3 - - Industrial/Textile Schedule for the United States Tariff Elimination US 8 digit Description MFN RATE Schedule 03011000 Live ornamental fish Free I 03019100 Live trout Free I 03019200 Live eels Free I 03019300 Live carp Free I 03019900 Live fish, other than trout, eel, carp or ornamental fish Free I 03021100 Trout, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I Pacific, Atlantic and Danube salmon, fresh or chilled, excluding fillets, other 03021200 meat portions, livers and roes Free I Salmonidae other than trout or Pacific, Atlantic & Danube salmon, fresh or 03021900 chilled, excluding fillets, other meat portions, livers & roes Free I Halibut and Greenland turbot, fresh or chilled, excluding fillets, other meat 03022100 portions, livers and roes Free I 03022200 Plaice, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I 03022300 Sole, fresh or chilled, excluding fillets, other meat portions, livers and roes 1.1 cents/kg A Flat fish, nesi, fresh or chilled, excluding fillets, other meat portions, livers 03022900 and roes Free I Albacore or longfinned tunas, fresh or chilled, excluding fillets, other meat 03023100 portions, livers and roes Free I Yellowfin tunas, fresh or chilled, excluding fillets, other meat portions, livers 03023200 and roes Free I Skipjack or stripe-bellied bonito, fresh or chilled, excluding fillets, other meat 03023300 portions, livers and roes Free -

PROVISIONAL PEER-REVIEWED TOXICITY VALUES for VANADIUM and ITS SOLUBLE INORGANIC COMPOUNDS OTHER THAN VANADIUM PENTOXIDE (CASRN 7440-62-2 and Others)
EPA/690/R-09/070F l Final 9-30-2009 Provisional Peer-Reviewed Toxicity Values for Vanadium and Its Soluble Inorganic Compounds Other Than Vanadium Pentoxide (CASRN 7440-62-2 and Others) Derivation of Subchronic and Chronic Oral RfDs Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 Commonly Used Abbreviations BMD Benchmark Dose IRIS Integrated Risk Information System IUR inhalation unit risk LOAEL lowest-observed-adverse-effect level LOAELADJ LOAEL adjusted to continuous exposure duration LOAELHEC LOAEL adjusted for dosimetric differences across species to a human NOAEL no-observed-adverse-effect level NOAELADJ NOAEL adjusted to continuous exposure duration NOAELHEC NOAEL adjusted for dosimetric differences across species to a human NOEL no-observed-effect level OSF oral slope factor p-IUR provisional inhalation unit risk p-OSF provisional oral slope factor p-RfC provisional inhalation reference concentration p-RfD provisional oral reference dose RfC inhalation reference concentration RfD oral reference dose UF uncertainty factor UFA animal to human uncertainty factor UFC composite uncertainty factor UFD incomplete to complete database uncertainty factor UFH interhuman uncertainty factor UFL LOAEL to NOAEL uncertainty factor UFS subchronic to chronic uncertainty factor i FINAL 9-30-2009 PROVISIONAL PEER-REVIEWED TOXICITY VALUES FOR VANADIUM AND ITS SOLUBLE INORGANIC COMPOUNDS OTHER THAN VANADIUM PENTOXIDE (CASRN 7440-62-2 and others) Background On December 5, 2003, the U.S. Environmental Protection Agency's (U.S. EPA) Office of Superfund Remediation and Technology Innovation (OSRTI) revised its hierarchy of human health toxicity values for Superfund risk assessments, establishing the following three tiers as the new hierarchy: 1) U.S. -

Calcium-Looping Performance of Steel and Blast Furnace Slags for Thermochemical Energy Storage in Concentrated Solar Power Plants
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by idUS. Depósito de Investigación Universidad de Sevilla Calcium-Looping performance of steel and blast furnace slags for Thermochemical Energy Storage in Concentrated Solar Power plants Jose Manuel Valverde a*, Juan Miranda-Pizarroa,c, Antonio Perejónb,c, Pedro E. Sánchez-Jiménezc, Luis A. Pérez-Maquedac aFacultad de Fisica, Universidad de Sevilla, Avenida Reina Mercedes s/n, 41012 Sevilla, Spain. bDepartamento de Química Inorgánica, Facultad de Química, Universidad de Sevilla, C. Prof. García González 1, Sevilla 41071, Spain. cInstituto de Ciencia de Materiales de Sevilla (C.S.I.C. - Universidad de Sevilla). C. Américo Vespucio 49, Sevilla 41092, Spain. Abstract The Calcium Looping (CaL) process, based on the carbonation/calcination of CaO, has been proposed as a feasible technology for Thermochemical Energy Storage (TCES) in Concentrated Solar Power (CSP) plants. The CaL process usually employs limestone as CaO precursor for its very low cost, non-toxicity, abundance and wide geographical distribution. However, the multicycle activity of limestone derived CaO under relevant CaL conditions for TCES in CSP plants can be severely limited by pore plugging. In this work, the alternative use of calcium-rich steel and blast furnace slags after treatment with acetic acid is investigated. A main observation is that the calcination temperature to regenerate the CaO is significantly reduced as compared to limestone. Furthermore, the multicycle activity of some of the slags tested at relevant CaL conditions for TCES remains high and stable if the treated samples are subjected to filtration. This process serves to remove silica grains, which helps decrease the porosity of the CaO resulting from calcination thus mitigating pore plugging. -

Calcination of Calcium Sulphoaluminate Cement Using Pyrite-Rich Cyanide Tailings
crystals Article Calcination of Calcium Sulphoaluminate Cement Using Pyrite-Rich Cyanide Tailings Kaiwei Dong 1, Feng Xie 1,*, Wei Wang 1,*, Yongfeng Chang 1, Chunlin Chen 2 and Xiaowei Gu 3 1 School of Metallurgy, Northeastern University, 3-11 Wenhua Road, Shenyang 110004, China; [email protected] (K.D.); [email protected] (Y.C.) 2 CSIRO Minerals Resources, Clayton, VIC 3168, Australia; [email protected] 3 Science and Technology Innovation Center of Smart Water and Resource Environment, Northeastern University, 3-11 Wenhua Road, Shenyang 110004, China; [email protected] * Correspondence: [email protected] (F.X.); [email protected] (W.W.); Tel.: +86-24-8367-2298 (F.X. & W.W.) Received: 23 September 2020; Accepted: 23 October 2020; Published: 26 October 2020 Abstract: Pyrite-rich cyanide tailings (CTs) are industrial hazardous solid wastes arising from the gold mining industry. Every year, hundreds of millions of tons of cyanide tailings are produced and discharged to tailings dams. It is of great significance to dispose of cyanide tailings harmlessly and resourcefully. The feasibility of calcination of calcium sulphoaluminate (CSA) cement clinker using pyrite-rich cyanide tailings as Fe2O3 and SO3 sources was investigated for this paper. The behavior of pyrite during the calcination of cyanide tailings under various calcination conditions and the properties of calcium sulphoaluminate cement clinker were examined. The results show that it is feasible to produce calcium sulphoaluminate cement clinker using pyrite-rich cyanide tailings. The optimal conditions for the calcination of calcium sulphoaluminate cement using pyrite-rich cyanide tailings are confirmed. -

Understanding Texts with the Help of Experimentation: the Example of Cupellation in Arabic Scientific Literature Sébastien Moureau, Nicolas Thomas
Understanding Texts with the Help of Experimentation: The Example of Cupellation in Arabic Scientific Literature Sébastien Moureau, Nicolas Thomas To cite this version: Sébastien Moureau, Nicolas Thomas. Understanding Texts with the Help of Experimentation: The Example of Cupellation in Arabic Scientific Literature. Ambix, Maney Publishing, 2016, 63 (2), pp.98 - 117. 10.1080/00026980.2016.1216691. hal-01766840 HAL Id: hal-01766840 https://hal-inrap.archives-ouvertes.fr/hal-01766840 Submitted on 14 Apr 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Ambix ISSN: 0002-6980 (Print) 1745-8234 (Online) Journal homepage: http://www.tandfonline.com/loi/yamb20 Understanding Texts with the Help of Experimentation: The Example of Cupellation in Arabic Scientific Literature Sébastien Moureau & Nicolas Thomas To cite this article: Sébastien Moureau & Nicolas Thomas (2016) Understanding Texts with the Help of Experimentation: The Example of Cupellation in Arabic Scientific Literature, Ambix, 63:2, 98-117, DOI: 10.1080/00026980.2016.1216691 To link to this article: http://dx.doi.org/10.1080/00026980.2016.1216691 Published online: 29 Sep 2016. Submit your article to this journal Article views: 16 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=yamb20 Download by: [Nicolas THOMAS] Date: 13 October 2016, At: 05:51 ambix, Vol. -

Mercury and Mercury Compounds
United States Office of Air Quality EPA-454/R-97-012 Environmental Protection Planning And Standards Agency Research Triangle Park, NC 27711 December 1997 AIR EPA LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF MERCURY AND MERCURY COMPOUNDS L & E EPA-454/R-97-012 Locating And Estimating Air Emissions From Sources of Mercury and Mercury Compounds Office of Air Quality Planning and Standards Office of Air and Radiation U.S. Environmental Protection Agency Research Triangle Park, NC 27711 December 1997 This report has been reviewed by the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, and has been approved for publication. Mention of trade names and commercial products does not constitute endorsement or recommendation for use. EPA-454/R-97-012 TABLE OF CONTENTS Section Page EXECUTIVE SUMMARY ................................................ xi 1.0 PURPOSE OF DOCUMENT .............................................. 1-1 2.0 OVERVIEW OF DOCUMENT CONTENTS ................................. 2-1 3.0 BACKGROUND ........................................................ 3-1 3.1 NATURE OF THE POLLUTANT ..................................... 3-1 3.2 OVERVIEW OF PRODUCTION, USE, AND EMISSIONS ................. 3-1 3.2.1 Production .................................................. 3-1 3.2.2 End-Use .................................................... 3-3 3.2.3 Emissions ................................................... 3-6 4.0 EMISSIONS FROM MERCURY PRODUCTION ............................. 4-1 4.1 PRIMARY MERCURY -

Nickel Laterite Smelting Processes and Some Examples of Recent Possible Modifications to the Conventional Route
metals Review Nickel Laterite Smelting Processes and Some Examples of Recent Possible Modifications to the Conventional Route Ender Keskinkilic Department of Metallurgical and Materials Engineering, Atilim University, 06830 Ankara, Turkey; [email protected]; Tel.: +90-533-302-9510 Received: 16 July 2019; Accepted: 1 September 2019; Published: 3 September 2019 Abstract: The treatment of laterites has been a research hotspot in extractive metallurgy over the past decades. Industrially, the pyrometallurgical treatment of laterites is mostly accomplished with a well-established method, namely, the rotary kiln–electric arc furnace (RKEF) process, which includes three main operations—calcination, prereduction, and smelting—followed by further refining for the removal of impurities from the raw ferro-nickel. As indicated in many studies of the RKEF process, the major downside of this method is its high energy consumption. Efforts have been made to lower this consumption. Furthermore, several new processes have been proposed. Among these, low-grade ferro-nickel production is regarded as the most widely and industrially used process after traditional RKEF operation. Although not widespread, other alternative processes of industrial scale have been generated since the start of the millennium. Recently, certain innovative processes have been tested either in the laboratory or at pilot-scale. In this paper, a literature review related to the smelting of laterites is made, and an emphasis on new processes and some examples of new developments in the RKEF process are presented. Keywords: laterite; smelting; RKEF process; low-grade ferro-nickel 1. Introduction According to a review published by Diaz et al., oxide-type nickel ores accounted for 64% of land-based nickel ores, but more than 60% of nickel production was based on the matte smelting of sulfide ores in 1988 [1].