Form 10-K Antares Pharma, Inc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Formulation and Evaluation of Transdermal Patch and Gel of Nateglinide
Human Journals Research Article September 2015 Vol.:4, Issue:2 © All rights are reserved by C. Aparna et al. Formulation and Evaluation of Transdermal Patch and Gel of Nateglinide Keywords: Nateglinide, transdermal patch and gel, HPMC, ethyl cellulose, carbopol, PVA, PVP ABSTRACT Anusha Gundeti, C. Aparna*, Dr. Prathima Srinivas The objective of the present work was to formulate Transdermal Drug Delivery systems of Nateglinide, an Department of Pharmaceutics, Sri Venkateshwara antidiabetic drug belonging to meglitinide class with a half life of 1.5 hrs. Transdermal patches containing nateglinide were College of Pharmacy, prepared by solvent casting method using the combinations Affiliated to Osmania University, of HPMC:EC, PVA:PVP, HPMC:Eudragit RS 100, Eudragit RL100:RS100 in different proportions and by incorporating Madhapur, Hyderabad, Telangana -500081, India. different permeation enhancers (polyethylene glycol 400, Su bmission: 7 September 2015 DMSO). The transdermal patches were evaluated for their physicochemical properties like thickness, weight variation, Accepted: 11 September 2015 folding endurance, percentage moisture absorption, percentage Published: 25 September 2015 moisture loss, in-vitro diffusion studies & ex-vivo permeation studies. Transdermal Gel was formulated using HPMC, carbopol 934, carbopol 940 and methyl cellulose. Gels were evaluated for homogeneity, pH, viscosity, drug content, in-vitro diffusion studies & ex-vivo permeation studies. By comparing the drug release F5 (HPMC:EC) formulation was selected as optimized formulation as it could sustain the drug release for 12 hrs i.e. 99.2% when compared to gel. Stability studies were www.ijppr.humanjournals.com carried out according to ICH guidelines and the patches maintained integrity and good physicochemical properties during the study period. -

Chrysler Affiliate Rewards Program
Chrysler Affiliate Rewards Program If you or your spouse work, or have retired from, one of the companies listed below, you may qualify for pricing as low as 1% Below Factory Invoice on a New FIAT! Check the list of companies below to see if your company qualifies.* A F O AAA-- State of Ohio Members Freightliner Of Tampa, Llc Ocala Freightliner ABB, Inc. Freightliner Of Toledo OCE'- North America Abbott Labs Freightliner Of Utah, Llc Ocean Freightliner, Ltd. Abbott, Nicholson, Quilter, Freightliner Trucks So. Florida Inc. O'connor Gmc, Inc. Esshaki & Youngblood PC Freightliner Twin Ports O'connor Truck Sales, Inc. Abercrombie & Fitch Freightmasters Ohio Machinery Company AboveNet Fresenius Medical dba Ohio CAT Abraxis Bioscience Inc. Fresno Truck Center Oklahoma City Freightliner Accor North America FRIENDLY MOTORCARS Oklahoma Farm Bureau Ace Hardware Corporation Fru-Con Construction Corporation Oklahoma Publishing Company, Action Western Star Fujisawa Healthcare Inc. The (OPUBCO) Actelion Pharmaceuticals US, Inc. Ftl And Ws Of Maine Old Dominion Freight Lines Action Couriers Ftl And Wst Of Tifton Oldcastle Inc. ADVANTAGE Health Solutions, Inc. Ftl Stl Wst Of Odessa Omaha Truck Center Inc Advance Publications Ftl Trucks Of South Florida Omni Care Health Plan Aearo Company Ftl, Stl And Wst Of Montgomery One Call Locators Aetna Ftl,Stl, and Western Star Of Dothan One Source Management Inc Affinia Group Fyda Freightliner Cincinnati Oracle Corporation Agar Truck Sales, Inc. Fyda Freightliner Columbus,Inc Organon Pharmaceuticals USA, Inc. AGCO Corporation Fyda Freightliner Pittsburgh Orlando Freightliner AGFA Corporation Fyda Freightliner Youngstown Orlando Freightliner South Aggreko, LLC ORRIN B HAYES, INC. Agricredit Acceptance LLC G Oscient Pharmaceuticals Agrilink Foods OSI Pharmaceuticals Gabrielli Ford Truck Sales AGSTAR Financial Services Otjen, Van Ert, Stangle, Lieb & Weir, S.C. -

Manufacturers with Signed Rebate Agreements February 1 , 2011
Wisconsin Medicaid Pharmacy Data Table Manufacturers with Signed Rebate Agreements February 1 , 2011 NEWLABELER NAME START END SC NEW LABELER NAME START END SC 00002 ELI LILLY AND COMPANY 1/1/1991 Y 00126 COLGATE ORAL PHARMACEUTICAL 1/1/1991 Y 00003 E R SQUIBB AND SONS INC. 1/1/1991 Y 00131 SCHWARZ PHARMA, INC. 1/1/1991 Y 00004 HOFFMANN LAROCHE INC 1/1/1991 00132 C B FLEET COMPANY INC. 1/1/1991 00005 LEDERLE LABORATORIES 1/1/1991 Y 00135 SMITHKLINE BEECHAM 1/1/1995 Y 00006 MERCK SHARP & DOHME 1/1/1991 Y 00143 WEST-WARD PHARMACEUTICAL C 1/1/1991 Y 00007 SMITHKLINE BEECHAM CORPORATI 1/1/1991 Y 00145 STIEFEL LABORATORIES INC. 1/1/1991 Y 00008 WYETH AYERST LABORATORIES 1/1/1991 Y 00149 PROCTER & GAMBLE PHARMACEU 1/1/1991 Y 00009 PHARMACIA AND UPJOHN 1/1/1991 Y 00168 E FOUGERA AND CO, DIV OF ALTAN 1/1/1991 Y 00013 PHARMACIA AND UPJOHN 1/1/1991 Y 00169 NOVO NORDISK PHARMACEUTICAL 1/1/1991 Y 00015 INVAMED, INC 1/1/1991 Y 00172 ZENITH LABORATORIES, INC 1/1/1991 Y 00023 ALLERGAN INC. 1/1/1991 Y 00173 GLAXOSMITHKLINE 1/1/1991 Y 00024 SANOFI SYNTHELABO 1/1/1991 Y 00178 MISSION PHARMACAL COMPANY 1/1/1991 Y 00025 PHARMACIA CORPORATION 1/1/1991 Y 00182 GOLDLINE LABORATORIES INC 1/1/1991 Y 00026 BAYER CORP PHARMACEUTICAL DI 1/1/1991 Y 00185 EON LABS MANUFACTURING, INC. 1/1/1991 Y 00028 NOVARTIS PHARMACEUTICALS 1/1/1991 Y 00186 ASTRAZENECA LP 1/1/1991 Y 00029 SMITHKLINE BEECHAM CORPORATI 1/1/1991 Y 00187 ICN PHARMACEUTICALS INC. -

An Archetype Swing in Transdermal Drug Delivery
Indo American Journal of Pharmaceutical Research, 2017 ISSN NO: 2231-6876 A COMPREHENSIVE REVIEW ON MICRONEEDLES - AN ARCHETYPE SWING IN TRANSDERMAL DRUG DELIVERY G. Ravi*, N. Vishal Gupta, M. P. Gowrav Department of Pharmaceutics, JSS College of Pharmacy, JSS University, Shri Shivarathreeshwara Nagara, Mysuru, Karnataka, India. ARTICLE INFO ABSTRACT Article history Transdermal drug delivery is the non-invasive delivery of medications through the skin Received 23/12/2016 surface into the systemic circulation. The advantage of transdermal drug delivery system is Available online that it is painless technique of administration of drugs. The advantage of transdermal drug 31/01/2017 delivery system is that it is painless technique of administration of drugs. Transdermal drug delivery system can improve the therapeutic efficacy and safety of the drugs because drug Keywords delivered through the skin at a predetermined and controlled rate. Due to the various Microneedles, biomedical benefits, it has attracted many researches. The barrier nature of stratumcorneum Hypodermic Needles, poses a danger to the drug delivery. By using microneedles, a pathway into the human body Transdermal, can be recognized which allow transportation of macromolecular drugs such as insulin or Stratumcorneum, vaccine. These microneedles only penetrate outer layers of the skin, exterior sufficient not to Patch. reach the nerve receptors of the deeper skin. Thus the microneedles supplement is supposed painless and reduces the infection and injuries. Researches from the past few years showed that microneedles have emerged as a novel carrier and considered to be effective for safe and improved delivery of the different drugs. Microneedles development is created a new pathway in the drug delivery field. -

Transdermal Nicotine Maintenance Attenuates the Subjective And
Neuropsychopharmacology (2004) 29, 991–1003 & 2004 Nature Publishing Group All rights reserved 0893-133X/04 $25.00 www.neuropsychopharmacology.org Transdermal Nicotine Maintenance Attenuates the Subjective and Reinforcing Effects of Intravenous Nicotine, but not Cocaine or Caffeine, in Cigarette-Smoking Stimulant Abusers 1 1 ,1,2 Bai-Fang X Sobel , Stacey C Sigmon and Roland R Griffiths* 1Department of Psychiatry and Behavioral Science, Johns Hopkins University School of Medicine, Baltimore, MD, USA; 2Department of Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA The effects of transdermal nicotine maintenance on the subjective, reinforcing, and cardiovascular effects of intravenously administered cocaine, caffeine, and nicotine were examined using double-blind procedures in nine volunteers with histories of using tobacco, caffeine, and cocaine. Each participant was exposed to two chronic drug maintenance phases (21 mg/day nicotine transdermal patch and placebo transdermal patch). Within each drug phase, the participant received intravenous injections of placebo, cocaine (15 and 30 mg/70 kg), caffeine (200 and 400 mg/70 kg), and nicotine (1.0 and 2.0 mg/70 kg) in mixed order across days. Subjective and cardiovascular data were collected before and repeatedly after drug or placebo injection. Reinforcing effects were also assessed after each injection with a Drug vs Money Multiple-Choice Form. Intravenous cocaine produced robust dose-related increases in subjective and reinforcing effects; these effects were not altered by nicotine maintenance. Intravenous caffeine produced elevations on several subjective ratings; nicotine maintenance did not affect these ratings. Under the placebo maintenance condition, intravenous nicotine produced robust dose-related subjective effects, with maximal increases similar to the high dose of cocaine; nicotine maintenance significantly decreased the subjective and reinforcing effects of intravenous nicotine. -

A Brief Review on Transdermal Patches
Organic and Medicinal Chemistry International Journal ISSN 2474-7610 Review Article Organic & Medicinal Chem IJ Volume 7 Issue 2 - June 2018 Copyright © All rights are reserved by Nidhi Sharma DOI: 10.19080/OMCIJ.2018.07.555707 A Brief Review on Transdermal Patches Nidhi Sharma* HIMT College of Pharmacy, Greater Noida, India Submission: May 12, 2018; Published: June 05, 2018 *Corresponding author: Nidhi Sharma, HIMT College of Pharmacy, Greater Noida, India, Email: Abstract healingTransdermal to an injured drug area delivery of the system body. was An presentedadvantage toof overcome a transdermal the difficulties drug delivery of drug route delivery over especiallyother types oral of route. delivery A transdermal system such patch as oral, is a topical,medicated i.v., adhesive i.m., etc. ispatch that that the patchis placed provides on the askin controlled to deliver release a specific of the dose medication of medication into the through patient, the usually skin and through into the either bloodstream. a porous It membrane promotes covering a reservoir of medication or through body heat melting thin layers of medication embedded in the adhesive. The main disadvantage to transdermal delivery systems stems from the fact that the skin is a very effective barrier, as a result, only medications whose molecules are small can easily penetrate the skin, so it can be delivered by this method. This review article describes the overall introduction of transdermal patches including type of transdermal patches, method of preparation of transdermal -
Transdermal Opioid Patches: Quick Reference Guide
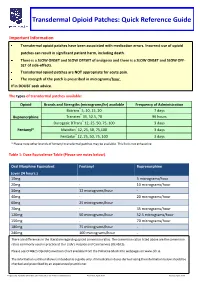
Transdermal Opioid Patches: Quick Reference Guide Important Information Transdermal opioid patches have been associated with medication errors. Incorrect use of opioid patches can result in significant patient harm, including death. There is a SLOW ONSET and SLOW OFFSET of analgesia and there is a SLOW ONSET and SLOW OFF- SET of side-effects. Transdermal opioid patches are NOT appropriate for acute pain. The strength of the patch is prescribed in micrograms/hour. If in DOUBT seek advice. The types of transdermal patches available: Opioid Brands and Strengths (micrograms/hr) available Frequency of Administration Butrans® 5, 10, 15, 20 7 days Buprenorphine Transtec® 35, 52.5, 70 96 hours Durogesic DTrans® 12, 25, 50, 75, 100 3 days Fentanyl* Matrifen® 12, 25, 50, 75,100 3 days Fentadur® 12, 25, 50, 75, 100 3 days * Please note other brands of fentanyl transdermal patches may be available. This list is not exhaustive. Table 1: Dose Equivalence Table (Please see notes below). Oral Morphine Equivalent Fentanyl Buprenorphine (over 24 hours.) 10mg 5 micrograms/hour 20mg - 10 micrograms/hour 30mg 12 micrograms/hour - 40mg - 20 micrograms/hour 60mg 25 micrograms/hour 70mg - 35 micrograms/hour 120mg 50 micrograms/hour 52.5 micrograms/hour 150mg - 70 micrograms/hour 180mg 75 micrograms/hour - 240mg 100 micrograms/hour - There are differences in the literature regarding opioid conversion ratios. The conversion ratios listed above are the conversion ratios commonly used in practice at Our Lady’s Hospice and Care Service (OLH&CS). Please see OLH&CS Opioid Conversion Chart available from the Palliative Meds Info webpages on www.olh.ie. -
Aptensio XR (Methylphenidate) Extended-Release Capsules
Aptensio XR™ (methylphenidate) – First-time authorized brand alternative • On May 5, 2020, Rhodes Pharmaceuticals launched an authorized brand alternative of its (Rhodes) branded Aptensio XR (methylphenidate) extended-release capsules. • Aptensio XR is approved for the treatment of attention deficit hyperactivity disorder (ADHD) in patients 6 years and older. • Methylphenidate is also available generically as an oral solution, extended-release capsule, extended-release tablet, tablet, and chewable tablet. It is also available as a brand extended-release capsule (Adhansia XR™, Jornay PM™), extended-release orally disintegrating tablet (Contempla-XR ODT™), transdermal patch (Daytrana®), extended-release chewable tablet (QuilliChew XR®), extended-release oral suspension (Quillivant XR®), and extended-release tablet (Relexxii™). — Methylphenidate oral solution, tablet, chewable tablet and extended-release tablets are approved to treat ADHD and narcolepsy. — Methylphenidate extended-release capsules, Adhansia XR, Contempla-XR ODT, Daytrana, Jornay PM, QuilliChew XR, Quillivant XR and Relexxii are approved to treat ADHD. • Aptensio XR carries a boxed warning for abuse and dependence. optumrx.com OptumRx® specializes in the delivery, clinical management and affordability of prescription medications and consumer health products. We are an Optum® company — a leading provider of integrated health services. Learn more at optum.com. All Optum® trademarks and logos are owned by Optum, Inc. All other brand or product names are trademarks or registered marks of their respective owners. This document contains information that is considered proprietary to OptumRx and should not be reproduced without the express written consent of OptumRx. RxNews® is published by the OptumRx Clinical Services Department. ©2020 Optum, Inc. All rights reserved. . -

ANTARES PHARMA, INC. 100 Princeton South, Suite 300 Ewing, New Jersey 08628 April 27, 2020 Dear Stockholder: You Are Cordially I
ANTARES PHARMA, INC. 100 Princeton South, Suite 300 Ewing, New Jersey 08628 April 27, 2020 Dear Stockholder: You are cordially invited to attend the 2020 Annual Meeting of Stockholders of Antares Pharma, Inc. (the “Company”), to be held at 10:00 a.m., Eastern Time, on Thursday, June 11, 2020 virtually via the Internet at www.virtualshareholdermeeting.com/ATRS2020 (the “Annual Meeting”). For the first time, our annual meeting will be a “virtual meeting” of stockholders, which will be conducted exclusively via the Internet through a virtual web conference. There will not be a physical meeting location, and stockholders will not be able to attend the annual meeting in person. Instructions on how to participate in the Annual Meeting and demonstrate proof of stock ownership are posted at www.virtualshareholdermeeting.com/ATRS2020. This means that you can attend the annual meeting online, vote your shares electronically and submit questions during the online meeting by visiting the above-mentioned website. We believe that hosting a “virtual meeting” will enable greater stockholder attendance and participation from any location around the world. In addition, in response to continued public health concerns related to COVID-19 and group gatherings, we also believe holding a “virtual meeting” will help ensure the health and well-being of our stockholders and other meeting participants. The notice of the 2020 Annual Meeting of Stockholders and the proxy statement that appear on the following pages describe the matters scheduled to come before the meeting. In addition, certain members of our Board of Directors and management team, as well as representatives of KPMG LLP, our independent registered public accounting firm, will be available to answer appropriate questions. -

Transdermal and Transbuccal Drug Delivery
TRANSDERMAL AND TRANSBUCCAL DRUG DELIVERY: ENHANCMENT USING IONTOPHORESIS AND CHEMICAL ENHANCERS by LONSHENG HU A Dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey in partial fulfillment of the requirements for the degree of Doctor of Philosophy Graduate Program in Pharmaceutical Science written under the direction of Bozena Michniak-Kohn and approved by ________________________ ________________________ ________________________ ________________________ New Brunswick, New Jersey October, 2010 ABSTRACT OF THE DISSERTATION TRANSDERMAL AND TRANSBUCCAL DRUG DELIVERY: ENHANCMENT USING IONTOPHORESIS AND CHEMICAL ENHANCERS By LONSHENG HU Dissertation Director: Professor Bozena Michniak-Kohn Transdermal and transbuccal routes offer attractive alternatives for systemic delivery of drugs due to their distinct advantages: non-invasive, avoidance of first-pass effect, improved bioavailability and reduction of systemic side effects. However, only a few drugs have been successfully delivered into blood stream to reach therapeutic levels without causing notable skin irritation or damage. Transbuccal drug delivery systems are still at research stage. The major barriers to transdermal and transbuccal drug delivery are stratum corneum of skin and epithelium of buccal tissue. The objective of this work was to overcome these barriers to significantly enhance transdermal and transbuccal delivery of hydrophilic drugs without causing major damage to skin and buccal tissue. In this work, iontophoresis, chemical -

Transdermal and Parenteral Fentanyl Dosage Calculations and Conversions
CHAPTER 5 Transdermal and Parenteral Fentanyl Dosage Calculations and Conversions OBJECTIVES INTRODUCTION After reading this chapter and completing Fentanyl is a synthetic pure mu opioid all practice problems, the participant will agonist with pharmacologic properties be able to: similar to morphine, hydromorphone, 1. Describe the pharmacokinetics of oxycodone and other opioids. Important transdermal fentanyl, and variables differences about fentanyl include its that can influence dosing. high degree of potency (about 75–100 times more potent than morphine on a 2. Recommend an appropriate dose of mg-to-mg basis), and greater lipid solubil- transdermal fentanyl when switching ity than morphine. The lipophilic nature from other opioids, including rescue of fentanyl facilitates rapid diffusion opioid dosing. The participant will be across the blood-brain barrier, resulting able to describe the appropriate tim- in a quick onset of action once the drug ing of this conversion. is absorbed from the administration site. 3. Recommend a strategy for switching Fentanyl is available in several dosage from transdermal fentanyl to another formulations, and may be administered opioid regimen, including dosing and by the following routes for a variety of appropriate timing. pain-related indications: 4. Describe how to transition between n Parenteral—fentanyl may be given by intravenous (IV) fentanyl and trans- intravenous (IV) injection, IV infu- dermal fentanyl. sion, subcutaneous (SQ) infusion or intramuscular (IM) injection (although we already agreed that an IM analge- sic is an oxymoron, and this practice is discouraged). It is used parenterally preoperatively, intraoperatively and postoperatively, and is occasionally used for the management of severe acute and chronic pain in other clinical situations. -

Pharmaceutical Company Contact Information (PDF)
Pharmaceutical Company Contact Information - Rebate Filing - as of June 2018 Labeler Name Invoice Contact Phone Extension 00002 LILLY USA, LLC LISA NORTON (317) 276-2000 00003 ER SQUIBB AND SONS INC. LYNN LEWIS (609) 897-4731 00004 GENENTECH CONTRACT ADMINISTRATION (650) 866-2666 00005 LEDERLE LABORATORIES DAN MAGUIRE (484) 563-5097 00006 MERCK & CO., INC. DOUG BICKFORD (215) 652-0671 00007 SMITHKLINE BEECHAM DAVID BUCKLEY (215) 751-5690 00008 WYETH LABORATORIES JENNIFER WOOTEN (901) 215-1883 00009 PHARMACIA AND UPJOHN COMPANY/PFIZER JENNIFER WOOTEN (901) 215-1883 00013 PHARMACIA AND UPJOHN COMPANY NICHOLAS CHRISTODOULOU (336) 291-1053 00014 G. D. SEARLE & CO. CINDY MCDONALD (847) 581-5726 00015 MEAD JOHNSON AND COMPANY LYNN LEWIS (609) 897-4731 00016 PHARMACIA INC. BARBARA WINGET (908) 901-7254 00023 ALLERGAN INC SHOBHANA MINAWALA (714) 246-6205 00024 SANOFI WINTHROP PHARMACEUTICALS LAURIE DUNLAP, ADMIN., GOVT. OPERATIONS (212) 551-4198 00025 PHARMACIA CORPORATION NICHOLAS CHRISTODOULOU (336) 291-1053 00026 BAYER CORPORATION PHARMACEUTICAL DIV. LINDA WOLCHESKI (203) 812-6372 00028 NOVARTIS PHARMACEUTICALS (862) 778-8094 00029 SMITHKLINE BEECHAM DAVID BUCKLEY (215) 751-5690 00031 A. H. ROBINS COMPANY DAN MAGUIRE (610) 902-3222 00032 SOLVAY PHARMACEUTICALS STACEY LENOX (847) 937-3979 00033 SYNTEX LABORATORIES, INC. JANICE BRENNAN (973) 562-3494 00034 THE PURDUE FREDERICK COMPANY JUNE STOWE (203) 899-8035 00037 CARTER-WALLACE, INC. JAY R BRENNAN (609) 655-6163 00038 ASTRAZENECA LP DAVID WRIGHT (302) 886-2268 7820 00039 AVENTIS PHARMACEUTICALS (908) 981-7461 00043 NOVARTIS CONSUMER HEALTH, INC. EDWARD D. COLLINS (973) 781-6191 00044 KNOLL LABORATORIES DEBRA DEYOUNG (847) 937-4372 00045 MCNEIL PHARMACEUTICAL (908) 218-6777 00046 AYERST LABORATORIES (901) 215-1473 00047 WARNER CHILCOTT LABORATORIES LISA KAROLCHYK (973) 442-3262 00048 KNOLL PHARMACEUTICAL COMPANY DEBRA DEYOUNG (847) 937-4372 00049 ROERIG NICHOLAS CHRISTODOULOU (336) 291-1053 00051 UNIMED PHARMACEUTICALS, INC STACY LENOX (847) 937-3979 00052 ORGANON, USA, INC.