Hematology Essentials: a Foundation for Accurate Smear Reviews
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reactive Plasmacytosis in a Patient of Acute Myeloid Leukemia at Initial
Open Journal of Clinical & Medical Volume 7 (2021) Case Reports Issue 01 ISSN: 2379-1039 Reactive plasmacytosis in a patient of acute myeloid leukemia at initial presentation – A rare occurrence Sundas Ali; Shahzad Ali Jiskani*; Samina Tufail Amanat; Aliena Sohail *Corresponding Author: Shahzad Ali Jiskani Department of Pathology, Pakistan Institute of Medical Sciences, Islamabad. Email: [email protected] Abstract An increased proliferation of plasma cells has been seen very rarely in patients of Acute Myeloid Leukemia (AML) at the time of initial presentation. In this report, we describe a 64-year old case of AML with reactive plasmacytosis at the time of diagnosis. It has been suggested that paraneoplastic IL-6 production by leuke- mic blasts may be responsible for growth stimulation of plasma cells in marrow. Keywords Acute myeloid leukemia; reactive plasmacytosis; paraneoplastic IL-6. Background The presence of reactive plasmacytosis in patients with acute myeloid leukemia has been shown after chemotherapy. However, it is a rare occurrence in a newly diagnosed AML case. Our patient presen- ted with a moderate proliferation of plasma cells in the bone marrow, along with morphological features consistent with the diagnosis of acute myeloid leukemia with maturation. A careful investigative approach is required to resolve this diagnostic dilemma [1,2]. Case presentation This 64-year old male patient presented to our hospital with complaints of fever, productive cough, generalized weakness and undocumented weight loss for the last one month, and epistaxis and hematuria for the last 5 days. He was a known case of HCV taking no treatment. Physical examination revealed only pallor and mild jaundice. -

The Hematological Complications of Alcoholism
The Hematological Complications of Alcoholism HAROLD S. BALLARD, M.D. Alcohol has numerous adverse effects on the various types of blood cells and their functions. For example, heavy alcohol consumption can cause generalized suppression of blood cell production and the production of structurally abnormal blood cell precursors that cannot mature into functional cells. Alcoholics frequently have defective red blood cells that are destroyed prematurely, possibly resulting in anemia. Alcohol also interferes with the production and function of white blood cells, especially those that defend the body against invading bacteria. Consequently, alcoholics frequently suffer from bacterial infections. Finally, alcohol adversely affects the platelets and other components of the blood-clotting system. Heavy alcohol consumption thus may increase the drinker’s risk of suffering a stroke. KEY WORDS: adverse drug effect; AODE (alcohol and other drug effects); blood function; cell growth and differentiation; erythrocytes; leukocytes; platelets; plasma proteins; bone marrow; anemia; blood coagulation; thrombocytopenia; fibrinolysis; macrophage; monocyte; stroke; bacterial disease; literature review eople who abuse alcohol1 are at both direct and indirect. The direct in the number and function of WBC’s risk for numerous alcohol-related consequences of excessive alcohol increases the drinker’s risk of serious Pmedical complications, includ- consumption include toxic effects on infection, and impaired platelet produc- ing those affecting the blood (i.e., the the bone marrow; the blood cell pre- tion and function interfere with blood cursors; and the mature red blood blood cells as well as proteins present clotting, leading to symptoms ranging in the blood plasma) and the bone cells (RBC’s), white blood cells from a simple nosebleed to bleeding in marrow, where the blood cells are (WBC’s), and platelets. -

Hematology Unit Lab 1 Review Material
Hematology Unit Lab 1 Review Material Objectives Laboratory instructors: 1. Facilitate lab discussion and answer questions Students: 1. Review the introductory material below 2. Study and review the assigned cases and questions in small groups before the Lab. This includes the pathological material using Virtual Microscopy 3. Be prepared to present your cases, questions and answers to the rest of your Lab class during the Lab Erythropoiesis: The process of red blood cell (RBC) production • Characterized by: − Increasing hemoglobin synthesis Erythroid maturation stages (Below): − Decreasing cell size - Average of 4 cell divisions during maturation − Decreasing cytoplasmic basophilia [One pronormoblast gives rise to 16 red cells] (increasing pink color) - pronormoblast → reticulocyte = 7 days − Progressive chromatin condensation of the - reticulocytes → mature RBC =1-2 days nuclei − Extrusion of nucleus (orthochromatic stage) − Extruded nuclei are subsequently phagocytized − Loss of mitotic capability after the early stage of polychromatophilic normoblast • Picture below: Erythroid progenitors (normoblasts) cluster around macrophages (arrows) in the bone marrow and spleen • Macrophages store iron • Iron is transferred from macrophages to erythroid precursor cells • Iron is used by normoblasts for hemoglobin synthesis aka nucleated rbc aka reticulocyte 1 Mature Red Blood Cell 7-8 microns; round / ovoid biconcave disc with orange-red cytoplasm, no RNA, no nucleus; survives ~120 days in circulation Classification of Anemia by Morphology 1. -

Complete Blood Count in Primary Care
Complete Blood Count in Primary Care bpac nz better medicine Editorial Team bpacnz Tony Fraser 10 George Street Professor Murray Tilyard PO Box 6032, Dunedin Clinical Advisory Group phone 03 477 5418 Dr Dave Colquhoun Michele Cray free fax 0800 bpac nz Dr Rosemary Ikram www.bpac.org.nz Dr Peter Jensen Dr Cam Kyle Dr Chris Leathart Dr Lynn McBain Associate Professor Jim Reid Dr David Reith Professor Murray Tilyard Programme Development Team Noni Allison Rachael Clarke Rebecca Didham Terry Ehau Peter Ellison Dr Malcolm Kendall-Smith Dr Anne Marie Tangney Dr Trevor Walker Dr Sharyn Willis Dave Woods Report Development Team Justine Broadley Todd Gillies Lana Johnson Web Gordon Smith Design Michael Crawford Management and Administration Kaye Baldwin Tony Fraser Kyla Letman Professor Murray Tilyard Distribution Zane Lindon Lyn Thomlinson Colleen Witchall All information is intended for use by competent health care professionals and should be utilised in conjunction with © May 2008 pertinent clinical data. Contents Key points/purpose 2 Introduction 2 Background ▪ Haematopoiesis - Cell development 3 ▪ Limitations of reference ranges for the CBC 4 ▪ Borderline abnormal results must be interpreted in clinical context 4 ▪ History and clinical examination 4 White Cells ▪ Neutrophils 5 ▪ Lymphocytes 9 ▪ Monocytes 11 ▪ Basophils 12 ▪ Eosinophils 12 ▪ Platelets 13 Haemoglobin and red cell indices ▪ Low haemoglobin 15 ▪ Microcytic anaemia 15 ▪ Normocytic anaemia 16 ▪ Macrocytic anaemia 17 ▪ High haemoglobin 17 ▪ Other red cell indices 18 Summary Table 19 Glossary 20 This resource is a consensus document, developed with haematology and general practice input. We would like to thank: Dr Liam Fernyhough, Haematologist, Canterbury Health Laboratories Dr Chris Leathart, GP, Christchurch Dr Edward Theakston, Haematologist, Diagnostic Medlab Ltd We would like to acknowledge their advice, expertise and valuable feedback on this document. -

Auer Rod-Like Inclusions and Hemophagocytosis in Neoplastic Cells of Multiple Myeloma
Hematology & Transfusion International Journal Case Report Open Access Auer rod-like inclusions and hemophagocytosis in neoplastic cells of multiple myeloma Abstract Volume 1 Issue 3 - 2015 Objective: Several intracytoplasmic morphological changes in the plasma cells of Juan Zhang,1 YanxinLi,1 Shunjun Li,1 Xianyong multiple myeloma have been described previously. However, Auer rod-like inclusions Jiang,2 Yucheng Meng,3 Mingyong Li,1 Yuan and hemophagocytosis are rarely found in these types of cells. In this paper, we intend 1 1 to report a rare case of multiple myeloma. He, Wenfang Huang 1Clinical Laboratory of Sichuan Academy of Medical Science & Methods: Bone marrow aspiration from the right superior iliac spine was examined Sichuan Provincial People’s Hospital, Chengdu, China twice. Cells were stained with May–Grünwald–Giemsa method. Bone scan 2Haematology bone marrow inspection laboratory of Peking demonstrated a focal lesion in the left iliac crest, which was confirmed subsequently Union Medical College Hospital, Beijing, China as a lytic lesion on CT scanning. By flow cytometry, plasma cells expressed CD38, 3Clinical Laboratory of Langfang Hospital of Traditional Chinese CD138, and CD56, CD184 and were negative for CD10, CD19, CD20, CD22, CD27 Medicine, Hebei, China and CyclinD1, with extensive strong Kappa light chain immunostaining. A complete Yanxin Li, Clinical Laboratory of Sichuan blood count and serum chemistry were also examined. Correspondence: Academy of Medical Science & Sichuan provincial People’s Results: It was -
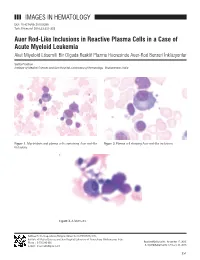
IMAGES in HEMATOLOGY Auer Rod-Like Inclusions in Reactive
IMAGES IN HEMATOLOGY DOI: 10.4274/tjh.2015.0399 Turk J Hematol 2016;33:351-352 Auer Rod-Like Inclusions in Reactive Plasma Cells in a Case of Acute Myeloid Leukemia Akut Miyeloid Lösemili Bir Olguda Reaktif Plazma Hücresinde Auer-Rod Benzeri İnklüzyonlar Sarita Pradhan Institute of Medical Sciences and Sum Hospital, Laboratory of Hematology, Bhubaneswar, India Figure 1. Myeloblasts and plasma cells containing Auer rod-like Figure 2. Plasma cell showing Auer rod-like inclusions. inclusions. Figure 3. A Mott cell. Address for Correspondence/Yazışma Adresi: Sarita PRADHAN, M.D., Institute of Medical Sciences and Sum Hospital, Laboratory of Hematology, Bhubaneswar, India Phone : 9 776 243 866 Received/Geliş tarihi: November 17, 2015 E-mail : [email protected] Accepted/Kabul tarihi: February 23, 2016 351 Pradhan S: Auer Rod-Like Inclusions in Plasma Cells Turk J Hematol 2016;33:351-352 A 61-year-old female presented with decreasing hemoglobin for Keywords: Auer rods, Acute myeloid leukemia, Plasma cells the past 6 months. She had a history of multiple transfusions in the recent past. Laboratory investigations showed hemoglobin Anahtar Sözcükler: Auer cismi, Akut miyeloid lösemi, Plazma of 8.6 g/dL, total blood leukocyte count of 1.13x109/L, and hücreleri platelets of 80x109/L with the presence of occasional circulating blasts. Bone marrow examination revealed the presence of Conflict of Interest: The author of this paper has no conflicts 63% myeloblasts with prominent Auer rods and mild reactive of interest, including specific financial interests, relationships, plasmacytosis (6%). Some of the plasma cells showed Auer and/or affiliations relevant to the subject matter or materials rod-like thin slender inclusions (Figures 1, 2, and 3). -

Laboratory Diagnosis Review
Laboratory Diagnosis Review Hematology Definition: The study of the three cellular elements of blood: Red Blood Cells (RBCs), White Blood Cells (WBCs), and Platelets Hemoglobin (Hgb or Hb): The oxygen carrying compound in RBCs Reference Range: Men 14-18 g/dL, Women 12-16 g/dL, Boy and girl levels are equal till age 11 Smoking increases, Pregnancy decreases, Capillary levels in newborns are higher than venous levels, Race, Position, and Time of day have minor effects, High WBCs may falsely raise Hgb. Below normal Hgb = anemia Red Blood Cell Count Reference Range: Men 4l5-6 million / cubic ml, Women4.0-5.5 million / ml3. Hematocrit (Hct): The ratio of RBCs to plasma Reference Range, Men 40%-54%, Women 37%-47%, Depends mostly on the number of RBCs but is slightly effected by the average RBC size, Not measured directly, but is calculated from the RBC count and the mean corpuscular volume (MCV). Increased by smoking, Decrease = anemia Useful Relationships: Hb X 3 = Hct RBCs (millions) X 3 = Hgb RBCs (millions) X 9 = Hct Wintrobe Indices: These indices are only significant if the RBCs, Hgb, and/or Hct. is abnormal MCV: Mean Corpuscular Volume MCH: Mean Corpuscular Hemoglobin MCHC: Mean Corpuscular Hemoglobin Concentration RDW: Red blood cell Distribution Width MCV: Reference Ranges: Men 80-95 fl (femtoliters), Women 81-99 fl (femto = 1 quadrillionth) Increased MCV = Macrocytosis, Decreased = Microcytosis MCV is increased by smoking, by B12 and/or folic acid deficiency, chronic liver disease, chronic alcoholism, Cardiorespiratory problems… Some macrocytic patients will not have macrocytosis MCV is decreased by iron deficiency, thalassemia, and anemia of chronic disease MCH: Reference Range 27-31 pg MCH is increased by Macrocytic anemias. -

10 11 Cyto Slides 81-85
NEW YORK STATE CYTOHEMATOLOGY PROFICIENCY TESTING PROGRAM Glass Slide Critique ~ November 2010 Slide 081 Diagnosis: MDS to AML 9 WBC 51.0 x 10 /L 12 Available data: RBC 3.39 x 10 /L 72 year-old female Hemoglobin 9.6 g/dL Hematocrit 29.1 % MCV 86.0 fL Platelet count 16 x 109 /L The significant finding in this case of Acute Myelogenous Leukemia (AML) was the presence of many blast forms. The participant median for blasts, all types was 88. The blast cells in this case (Image 081) are large, irregular in shape and contain large prominent nucleoli. It is difficult to identify a blast cell as a myeloblast without the presence of an Auer rod in the cytoplasm. Auer rods were reported by three participants. Two systems are used to classify AML into subtypes, the French- American-British (FAB) and the World Health Organization (WHO). Most are familiar with the FAB classification. The WHO classification system takes into consideration prognostic factors in classifying AML. These factors include cytogenetic test results, patient’s age, white blood cell count, pre-existing blood disorders and a history of treatment with chemotherapy and/or radiation therapy for a prior cancer. The platelet count in this case was 16,000. Reduced number of platelets was correctly reported by 346 (94%) of participants. Approximately eight percent of participants commented that the red blood cells in this case were difficult to evaluate due to the presence of a bluish hue around the red blood cells. Comments received included, “On slide 081 the morphology was difficult to evaluate since there was a large amount of protein surrounding RBC’s”, “Slide 081 unable to distinguish red cell morphology due to protein” and “Unable to adequately assess morphology on slide 081 due to poor stain”. -

Advanced Blood Cell Id: Peripheral Blood Findings in Sickle Cell Anemia
ADVANCED BLOOD CELL ID: PERIPHERAL BLOOD FINDINGS IN SICKLE CELL ANEMIA Educational commentary is provided for participants enrolled in program #259- Advanced Blood Cell Identification. This virtual blood cell identification program includes case studies with more difficult challenges. To view the blood cell images in more detail, click on the sample identification numbers underlined in the paragraphs below. This will open a virtual image of the selected cell and the surrounding fields. If the image opens in the same window as the commentary, saving the commentary PDF and opening it outside your browser will allow you to switch between the commentary and the images more easily. Click on this link for the API ImageViewerTM Instructions. Learning Outcomes After completing this exercise, participants should be able to: • describe morphologic features of normal peripheral blood leukocytes. • identify morphologic characteristics distinctive of sickle cells. • distinguish selected RBC inclusions based on morphologic features. • describe significant morphologic characteristics of nucleated red blood cells. Case Study The CBC from a 30 year old African American male is as follows: WBC=9.5 x 109/L, RBC=1.66 x 1012/L, Hgb=5.0 g/dL, Hct=13.9%, MCV=83.7 fL, MCH=30.1 pg, MCHC=36.0 g/dL, RDW-CV=24.9%, MPV=9.6 fL, Platelet=326 x 109/L. Educational Commentary The cells and RBC inclusions chosen for identification in this testing event were seen in the peripheral blood of a man with a severe anemia resulting from sickle cell disease. The cell shown in ABI-08 contains a Howell-Jolly body. -

Electron Microscopic and Peroxidase Cytochemical Analysis of Pink Pseudo-Chediak-Higashi Granules in Acute Myelogenous Leukemia1
[CANCER RESEARCH 40, 4473-4481, December 1980] 0008-5472/80/0040-4473S02.00 Electron Microscopic and Peroxidase Cytochemical Analysis of Pink Pseudo-Chediak-Higashi Granules in Acute Myelogenous Leukemia1 William A. Dittman, Robert J. Kramer, and Dorothy F. Bainton2 Sacred Heart Medical Center, Spokane. Washington 99024 [W. A. D.]; Station 5. Pasco. Washington 99301 ¡R.J. K.J; and Department of Pathology. University of California School of Medicine. San Francisco. California 94143 ¡D.F. B.¡ ABSTRACT We have had the opportunity to study 3 patients with AML whose blasts, promyelocytes, and myelocytes contained enor Giant round pink inclusions (=2 jam)were seen in neutrophilic mous round pink inclusions, initially thought to be ingested myeloblasts, promyelocytes, and myelocytes from three pa RBC. Analysis by electron microscopy and peroxidase cyto tients with acute myelogenous leukemia. On preliminary ex chemistry revealed that these pink structures were large per amination of the bone marrow smears, these inclusions looked oxidase-positive granules and therefore also represented ab like ingested red blood cells in that they were pink and not normal variants of the azurophilic (primary) granule population. azurophilic. The bone marrow specimens were processed for In addition, these 3 cases did not have DIC. the electron microscopic demonstration of peroxidase with 3,3'-diaminobenzidine and H2C>2at pH 7.6. In all three cases, the inclusions were determined to be large peroxidase-positive MATERIALS AND METHODS granules since they were limited by a single unit membrane Case Studies and, unlike endocytized red blood cells, were not contained within phagocytic vacuoles. The granules were homogeneously Case 1. -

Advanced Blood Cell Identification
ADVANCED BLOOD CELL ID: LEUKOCYTES AND ERYTHROCYTES IN AN ACUTE LEUKEMIA Educational commentary is provided for participants enrolled in program #259- Advanced Blood Cell Identification. This virtual blood cell identification program includes case studies with more difficult challenges. To view the blood cell images in more detail, click on the sample identification numbers underlined in the paragraphs below. This will open a virtual image of the selected cell and the surrounding fields. If the image opens in the same window as the commentary, saving the commentary PDF and opening it outside your browser will allow you to switch between the commentary and the images more easily. Click on this link for the API ImageViewerTM Instructions. Learning Outcomes After completing this exercise, participants should be able to: • Discuss morphologic characteristics of normal peripheral blood leukocytes. • Describe morphologic features of immature granulocytes. • Identify morphologic abnormalities in erythrocyte shape and chromaticity/coloration. Case Study An 18 year old male was seen by his physician for bruising and severe nosebleeds. His CBC results are as follows: WBC=7.5 x 109/L, RBC=2.79 1012/L, Hgb=8.7 g/dL, Hct=24.6%, MCV=88 fL, MCH=31 pg, MCHC=35 g/dL, RDW=18.4%, Platelet=41 x 109/L, MPV=10.4. Educational Commentary The cells selected for identification and discussion in this exercise are from the peripheral blood smear of an 18 year old man diagnosed with acute promyelocytic leukemia (APL). APL is also referred to as acute myeloid leukemia, M3 (AML-M3). As with other acute leukemias, APL has a rapid onset. -

Morphologic Evaluation of Anemia – I Adewoyin S
nd M y a ed g ic lo i o n i e B Adewoyin and Ogbenna, Biol Med (Aligarh) 2016, 8:6 DOI: 10.4172/0974-8369.1000322 ISSN: 0974-8369 Biology and Medicine Review Article Open Access Morphologic Evaluation of Anemia – I Adewoyin S. Ademola1* and Ogbenna A. Abiola2 1Department of Haematology and Blood Transfusion, University of Benin Teaching Hospital, PMB 1111, Ugbowo, Benin City, Nigeria 2Department of Haematology and Blood Transfusion, Lagos University Teaching Hospital, Idi-Araba, Lagos, Nigeria *Corresponding author: Adewoyin S. Ademola, Department of Haematology and Blood Transfusion, University of Benin Teaching Hospital, PMB 1111, Ugbowo, Benin City, Nigeria, Tel: +2347033966347; E-mail: [email protected] Received date: June 20, 2016; Accepted date: July 15, 2016; Published date: July 22, 2016 Copyright: © 2016 Adewoyin AS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited. Abstract Anaemia is a feature of many tropical diseases. Anaemia diagnosis therefore remains a crucial intervention among physicians in developing countries. A barrage of laboratory test (anaemic work-up) is usually deployed in differentiating its underlying cause. However, central to anaemia evaluation is the morphology of the red cells and other cell lines. Conventionally, initial laboratory tests include full blood count, reticulocyte count and peripheral blood film (PBF). PBF is often a clinical request, performed by skilled technologist and reported by haematologist/ haematomorphologist. Findings from PBF are reviewed and reported in the light of patient’s clinical history and examination findings.