AML to DCMA CME Committee
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reactive Plasmacytosis in a Patient of Acute Myeloid Leukemia at Initial
Open Journal of Clinical & Medical Volume 7 (2021) Case Reports Issue 01 ISSN: 2379-1039 Reactive plasmacytosis in a patient of acute myeloid leukemia at initial presentation – A rare occurrence Sundas Ali; Shahzad Ali Jiskani*; Samina Tufail Amanat; Aliena Sohail *Corresponding Author: Shahzad Ali Jiskani Department of Pathology, Pakistan Institute of Medical Sciences, Islamabad. Email: [email protected] Abstract An increased proliferation of plasma cells has been seen very rarely in patients of Acute Myeloid Leukemia (AML) at the time of initial presentation. In this report, we describe a 64-year old case of AML with reactive plasmacytosis at the time of diagnosis. It has been suggested that paraneoplastic IL-6 production by leuke- mic blasts may be responsible for growth stimulation of plasma cells in marrow. Keywords Acute myeloid leukemia; reactive plasmacytosis; paraneoplastic IL-6. Background The presence of reactive plasmacytosis in patients with acute myeloid leukemia has been shown after chemotherapy. However, it is a rare occurrence in a newly diagnosed AML case. Our patient presen- ted with a moderate proliferation of plasma cells in the bone marrow, along with morphological features consistent with the diagnosis of acute myeloid leukemia with maturation. A careful investigative approach is required to resolve this diagnostic dilemma [1,2]. Case presentation This 64-year old male patient presented to our hospital with complaints of fever, productive cough, generalized weakness and undocumented weight loss for the last one month, and epistaxis and hematuria for the last 5 days. He was a known case of HCV taking no treatment. Physical examination revealed only pallor and mild jaundice. -

Auer Rod-Like Inclusions and Hemophagocytosis in Neoplastic Cells of Multiple Myeloma
Hematology & Transfusion International Journal Case Report Open Access Auer rod-like inclusions and hemophagocytosis in neoplastic cells of multiple myeloma Abstract Volume 1 Issue 3 - 2015 Objective: Several intracytoplasmic morphological changes in the plasma cells of Juan Zhang,1 YanxinLi,1 Shunjun Li,1 Xianyong multiple myeloma have been described previously. However, Auer rod-like inclusions Jiang,2 Yucheng Meng,3 Mingyong Li,1 Yuan and hemophagocytosis are rarely found in these types of cells. In this paper, we intend 1 1 to report a rare case of multiple myeloma. He, Wenfang Huang 1Clinical Laboratory of Sichuan Academy of Medical Science & Methods: Bone marrow aspiration from the right superior iliac spine was examined Sichuan Provincial People’s Hospital, Chengdu, China twice. Cells were stained with May–Grünwald–Giemsa method. Bone scan 2Haematology bone marrow inspection laboratory of Peking demonstrated a focal lesion in the left iliac crest, which was confirmed subsequently Union Medical College Hospital, Beijing, China as a lytic lesion on CT scanning. By flow cytometry, plasma cells expressed CD38, 3Clinical Laboratory of Langfang Hospital of Traditional Chinese CD138, and CD56, CD184 and were negative for CD10, CD19, CD20, CD22, CD27 Medicine, Hebei, China and CyclinD1, with extensive strong Kappa light chain immunostaining. A complete Yanxin Li, Clinical Laboratory of Sichuan blood count and serum chemistry were also examined. Correspondence: Academy of Medical Science & Sichuan provincial People’s Results: It was -
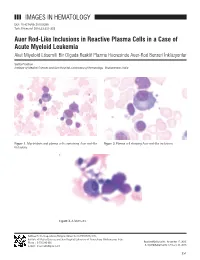
IMAGES in HEMATOLOGY Auer Rod-Like Inclusions in Reactive
IMAGES IN HEMATOLOGY DOI: 10.4274/tjh.2015.0399 Turk J Hematol 2016;33:351-352 Auer Rod-Like Inclusions in Reactive Plasma Cells in a Case of Acute Myeloid Leukemia Akut Miyeloid Lösemili Bir Olguda Reaktif Plazma Hücresinde Auer-Rod Benzeri İnklüzyonlar Sarita Pradhan Institute of Medical Sciences and Sum Hospital, Laboratory of Hematology, Bhubaneswar, India Figure 1. Myeloblasts and plasma cells containing Auer rod-like Figure 2. Plasma cell showing Auer rod-like inclusions. inclusions. Figure 3. A Mott cell. Address for Correspondence/Yazışma Adresi: Sarita PRADHAN, M.D., Institute of Medical Sciences and Sum Hospital, Laboratory of Hematology, Bhubaneswar, India Phone : 9 776 243 866 Received/Geliş tarihi: November 17, 2015 E-mail : [email protected] Accepted/Kabul tarihi: February 23, 2016 351 Pradhan S: Auer Rod-Like Inclusions in Plasma Cells Turk J Hematol 2016;33:351-352 A 61-year-old female presented with decreasing hemoglobin for Keywords: Auer rods, Acute myeloid leukemia, Plasma cells the past 6 months. She had a history of multiple transfusions in the recent past. Laboratory investigations showed hemoglobin Anahtar Sözcükler: Auer cismi, Akut miyeloid lösemi, Plazma of 8.6 g/dL, total blood leukocyte count of 1.13x109/L, and hücreleri platelets of 80x109/L with the presence of occasional circulating blasts. Bone marrow examination revealed the presence of Conflict of Interest: The author of this paper has no conflicts 63% myeloblasts with prominent Auer rods and mild reactive of interest, including specific financial interests, relationships, plasmacytosis (6%). Some of the plasma cells showed Auer and/or affiliations relevant to the subject matter or materials rod-like thin slender inclusions (Figures 1, 2, and 3). -
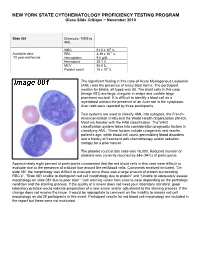
10 11 Cyto Slides 81-85
NEW YORK STATE CYTOHEMATOLOGY PROFICIENCY TESTING PROGRAM Glass Slide Critique ~ November 2010 Slide 081 Diagnosis: MDS to AML 9 WBC 51.0 x 10 /L 12 Available data: RBC 3.39 x 10 /L 72 year-old female Hemoglobin 9.6 g/dL Hematocrit 29.1 % MCV 86.0 fL Platelet count 16 x 109 /L The significant finding in this case of Acute Myelogenous Leukemia (AML) was the presence of many blast forms. The participant median for blasts, all types was 88. The blast cells in this case (Image 081) are large, irregular in shape and contain large prominent nucleoli. It is difficult to identify a blast cell as a myeloblast without the presence of an Auer rod in the cytoplasm. Auer rods were reported by three participants. Two systems are used to classify AML into subtypes, the French- American-British (FAB) and the World Health Organization (WHO). Most are familiar with the FAB classification. The WHO classification system takes into consideration prognostic factors in classifying AML. These factors include cytogenetic test results, patient’s age, white blood cell count, pre-existing blood disorders and a history of treatment with chemotherapy and/or radiation therapy for a prior cancer. The platelet count in this case was 16,000. Reduced number of platelets was correctly reported by 346 (94%) of participants. Approximately eight percent of participants commented that the red blood cells in this case were difficult to evaluate due to the presence of a bluish hue around the red blood cells. Comments received included, “On slide 081 the morphology was difficult to evaluate since there was a large amount of protein surrounding RBC’s”, “Slide 081 unable to distinguish red cell morphology due to protein” and “Unable to adequately assess morphology on slide 081 due to poor stain”. -

Electron Microscopic and Peroxidase Cytochemical Analysis of Pink Pseudo-Chediak-Higashi Granules in Acute Myelogenous Leukemia1
[CANCER RESEARCH 40, 4473-4481, December 1980] 0008-5472/80/0040-4473S02.00 Electron Microscopic and Peroxidase Cytochemical Analysis of Pink Pseudo-Chediak-Higashi Granules in Acute Myelogenous Leukemia1 William A. Dittman, Robert J. Kramer, and Dorothy F. Bainton2 Sacred Heart Medical Center, Spokane. Washington 99024 [W. A. D.]; Station 5. Pasco. Washington 99301 ¡R.J. K.J; and Department of Pathology. University of California School of Medicine. San Francisco. California 94143 ¡D.F. B.¡ ABSTRACT We have had the opportunity to study 3 patients with AML whose blasts, promyelocytes, and myelocytes contained enor Giant round pink inclusions (=2 jam)were seen in neutrophilic mous round pink inclusions, initially thought to be ingested myeloblasts, promyelocytes, and myelocytes from three pa RBC. Analysis by electron microscopy and peroxidase cyto tients with acute myelogenous leukemia. On preliminary ex chemistry revealed that these pink structures were large per amination of the bone marrow smears, these inclusions looked oxidase-positive granules and therefore also represented ab like ingested red blood cells in that they were pink and not normal variants of the azurophilic (primary) granule population. azurophilic. The bone marrow specimens were processed for In addition, these 3 cases did not have DIC. the electron microscopic demonstration of peroxidase with 3,3'-diaminobenzidine and H2C>2at pH 7.6. In all three cases, the inclusions were determined to be large peroxidase-positive MATERIALS AND METHODS granules since they were limited by a single unit membrane Case Studies and, unlike endocytized red blood cells, were not contained within phagocytic vacuoles. The granules were homogeneously Case 1. -

Advanced Blood Cell Identification
ADVANCED BLOOD CELL ID: LEUKOCYTES AND ERYTHROCYTES IN AN ACUTE LEUKEMIA Educational commentary is provided for participants enrolled in program #259- Advanced Blood Cell Identification. This virtual blood cell identification program includes case studies with more difficult challenges. To view the blood cell images in more detail, click on the sample identification numbers underlined in the paragraphs below. This will open a virtual image of the selected cell and the surrounding fields. If the image opens in the same window as the commentary, saving the commentary PDF and opening it outside your browser will allow you to switch between the commentary and the images more easily. Click on this link for the API ImageViewerTM Instructions. Learning Outcomes After completing this exercise, participants should be able to: • Discuss morphologic characteristics of normal peripheral blood leukocytes. • Describe morphologic features of immature granulocytes. • Identify morphologic abnormalities in erythrocyte shape and chromaticity/coloration. Case Study An 18 year old male was seen by his physician for bruising and severe nosebleeds. His CBC results are as follows: WBC=7.5 x 109/L, RBC=2.79 1012/L, Hgb=8.7 g/dL, Hct=24.6%, MCV=88 fL, MCH=31 pg, MCHC=35 g/dL, RDW=18.4%, Platelet=41 x 109/L, MPV=10.4. Educational Commentary The cells selected for identification and discussion in this exercise are from the peripheral blood smear of an 18 year old man diagnosed with acute promyelocytic leukemia (APL). APL is also referred to as acute myeloid leukemia, M3 (AML-M3). As with other acute leukemias, APL has a rapid onset. -

Auer Rod-Like Crystal Inclusions in Plasma Cells of Multiple Myeloma Sung-Hee Oh, Chan Jeoung Park
DOI: 10.5045/kjh.2010.45.4.222 The Korean Journal of Hematology Volume 45ㆍNumber 4ㆍDecember 2010 Auer rod-like crystal inclusions in plasma cells of multiple myeloma Sung-hee Oh, Chan Jeoung Park Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea A 65-year-old man with 10-year history of non-insulin dependent diabetes mellitus presented with anemia and thrombocytopenia, which lasted for 3 months. Blood cell counts were: WBC, 5.1×109/L; hemoglobin, 9.0 g/dL; plate- let, 64×109/L. Peripheral blood film showed no rouleaux formation. Routine chemistry showed: calcium, 9.4 mg/dL; blood urea nitrogen/creatinine, 12/1.1 mg/dL; protein/albumin, 6.3/3.5 g/dL. Radiologic studies showed suspicious os- teolytic lesions on the humerus and distal clavicles. Serum protein electrophoresis revealed M-peak of 0.2 g/dL, and immunofixation electrophoresis showed a zone of restriction in the kappa light chain, suggesting monoclonal component. Urine protein electrophoresis revealed free kappa-type Bence-Jones proteinuria (59.3% of urine protein). Bone marrow aspirates showed many plasma cells (64.0% of nucleated cells) with Auer rod-like crystal inclusions in the cytoplasm. (A) Engulfed Auer rod-like inclusions in numerous histiocytes and free Auer rod-like inclusions in the backgrounds were observed. (B) Bone marrow biopsy showed decreased cellularity with interstitial and nodular infiltration of plasma cells. Immunohistochemical findings confirmed the kappa monoclonality of plasma cells. Despite 2 cycles of dexamethasone treatment without complications, the patient still suffers from Bence-Jones proteinuria and worsened bone pain. -

11. Hematology
PBW11 2/6/03 1:49 PM Page 185 11. Hematology I. Introduction A. EMBRYOLOGY 1. Hematopoiesis begins in yolk sac during first month 2. At third month it begins in liver & spleen 3. Fourth month hematopoiesis begins in the bone marrow 4. At birth, liver & spleen hematopoiesis ØØ B. HEMATOPOIESIS 1. 3 stages: 1) proliferation of stem cells, 2) differentiation of blast cells, 3) maturation to final cell type 2. Common stem cell (preblast) expresses CD34 surface protein 3. CD34+ cells differentiate into 1) myeloid & 2) lymphoid blasts 4. Mutations in stem cell Æ myeloproliferative dz [see Section V] 5. Mutations in blast cell Æ acute leukemias [see Section VI] 6. Myelopoiesis a. Myeloblast differentiates Æ progenitors of 1) erythrocyte, 2) megakaryocyte, 3) mast cell, 4) monocyte & 5) granulocyte b. Granulocyte matures Æ neutrophil, eosinophil, basophil c. 90% of neutrophils die within marrow, last 6hr in circulation d. Monocyte circulates for 6–10hr, then matures in tissue e. Megakaryocytes become multinucleate due to endomitotic reduplication (nucleus multiplies without cell division), matured cells split off platelets, which circulate for 7 days f. Proerythrocyte nucleus shrinks & then is extruded, with Wright stain cytoplasm first Æ blue during RNA transcription, then Æ pink as hemoglobin is translated g. Mature RBCs circulate for 120 days 7. Lymphoid blast matures Æ B cells, T cells, natural killer cells (See Color Plate 14) C. DISORDERS 1. ≠ risk infection if absolute neutrophil count <1000/mm3 2. All blood cell disorders are of 3 general types a. Altered production: clonal proliferation or bone marrow failure b. Altered destruction: can be ≠ or Ø (Ø apoptosis Æ cancer) c. -

Containing Myeloma Cells in Patients with Newly
Cancer Medicine Open Access ORIGINAL RESEARCH Clinical significance of granule-containing myeloma cells in patients with newly diagnosed multiple myeloma Kazuhito Suzuki1, Shingo Yano1, Kaichi Nishiwaki1, Koji Sano1, Takaki Shimada1, Yuichi Yahagi1, Yoji Ogasawara1, Katsuki Sugiyama1, Shinobu Takahara1, Takeshi Saito1, Kinuyo Kasama1, Jiro Minami1, Hiroki Yokoyama1, Yutaro Kamiyama1, Atsushi Katsube1, Hidekazu Masuoka1, Mitsuji Katori1, Tomohito Machishima1, Aya Ouchi1, Nobuaki Dobashi1, Ken Kaito2, Noriko Usui1,3 & Keisuke Aiba1 1Division of Clinical Oncology/Hematology, Department of Internal Medicine, The Jikei University School of Medicine, Tokyo, Japan 2Central Clinical Laboratory, The Jikei University Hospital, Tokyo, Japan 3Division of Transfusion Medicine, The Jikei University School of Medicine, Tokyo, Japan Keywords Abstract CD49e, CD56, granules, morphology, myeloma, prognosis The clinical features and prognostic significance of myeloma cells containing granules remain unclear. The purpose of this retrospective study was to inves- Correspondence tigate the clinical significance of granule-containing myeloma cells in patients Kazuhito Suzuki, Division of Clinical with newly diagnosed multiple myeloma (NDMM). We retrospectively analyzed Oncology/Hematology, The Jikei University the records of 122 patients diagnosed with NDMM between January 2007 and School of Medicine, 3-25-8 Nishi-Shinbashi, December 2013. Granule-containing myeloma cells were defined as myeloma Minato-ku, Tokyo 105-8461, Japan. Tel: +81-3-3433-1111; cells that exhibited three or more granules in their cytoplasm by May-Giemsa Fax: +81-3-3436-3233; staining. The patients were classified into two groups, the granule-containing E-mail: [email protected] myeloma (GM) and nongranule-containing myeloma (non-GM) groups, depend- ing on the proportion of myeloma cells that contained granules (cut-off value: Funding Information 10%). -
Oxford Handbook of Clinical Haematology
https://kat.cr/user/tahir99/ Haematological emergencies Septic shock/neutropenic fever E p636 Acute transfusion reactions E p638 Delayed transfusion reaction E p642 Post-transfusion purpura E p643 Hypercalcaemia E p644 Hyperviscosity E p646 Disseminated intravascular coagulation E p648 Overdosage of thrombolytic therapy E p651 Heparin overdosage E p652 Heparin-induced thrombocytopenia (HIT) E p654 Warfarin overdosage E p656 Massive blood transfusion E p658 Paraparesis/spinal collapse E p662 Leucostasis E p663 Thrombotic thrombocytopenic purpura E p664 Sickle crisis E p666 Tumour lysis syndrome (TLS) E p690 https://kat.cr/user/tahir99/ oxford medical publications Oxford Handbook of Clinical Haematology https://kat.cr/user/tahir99/ Published and forthcoming Oxford Handbooks Oxford Handbook for the Foundation Programme 4/e Oxford Handbook of Acute Medicine 3/e Oxford Handbook of Anaesthesia 2/e Oxford Handbook of Applied Dental Sciences Oxford Handbook of Cardiology 2/e Oxford Handbook of Clinical and Laboratory Investigation 2/e Oxford Handbook of Clinical Dentistry 6/e Oxford Handbook of Clinical Diagnosis 3/e Oxford Handbook of Clinical Examination and Practical Skills 2/e Oxford Handbook of Clinical Haematology 4/e Oxford Handbook of Clinical Immunology and Allergy 3/e Oxford Handbook of Clinical Medicine—Mini Edition 9/e Oxford Handbook of Clinical Medicine 9/e Oxford Handbook of Clinical Pharmacy Oxford Handbook of Clinical Rehabilitation 2/e Oxford Handbook of Clinical Specialties 9/e Oxford Handbook of Clinical Surgery 4/e Oxford -

Chronic Lymphocytic Leukaemia
put together by Alex Yartsev: Sorry if i used your images or data and forgot to reference you. Tell me who you are. [email protected] Chronic Lymphocytic Leukaemia 4.02 Detailed History of Presenting Illness – Leukaemia in general HPI: PI: - Fatigue - Frequent Infections - Weakness - Past Radio/Chemotherapy - Malaise - Past Cancers - Fever Family/Social: - Nightsweats - Leucaemia - Weight loss - Jaundice - Smoking - Lymphadenopathy - Alcohol - Bone pain - CURRENT MEDICATIONS - Excessive bruising - Abdominal pain/swelling/”fullness” Patient’s AGE speaks volumes: !! IMPORTANT: get IIIMMMMUUNNIIISSAATTIIIOONN history !! - The YOUNG get ALL Differential Diagnoses (DDx) - The OLD get CLL + AML - Chronic Infection - Non-leukaemia cancer - Everyone gets - Hypersplenism everything else - Paraneoplastic GM-CSF production - Lymphoma - Anaemia - Depression Pertinent findings on Examination Leukaemia in General Any Leukaemia AML Only - Pallor or Jaundice - swollen, painful, and bleeding gums - - splenomegaly mets to the oral tissue; - hepatomegaly - pigmented (colored) rash-like spots - - abdominal swelling. mets to the skin; or - Lymphadenopathy - chloromas (granulocytic sarcomas; Advanced: Mets Brain collections of tumorous cells within the - central nervous system effects: skin or other body parts) - headaches - ecchymoses, epistaxis, or menorrhagia - seizures - weakness - blurred vision The T-cell variety of (ALL) may cause the - balance difficulties thymus to enlarge and press on the trachea - vomiting or the superior vena cava . -

Interpreting an Abnormal CBC
Interpreting an Abnormal CBC Nicholas A. Forward MD, MSc, FRCPC Assistant Professor Division of Hematology Dalhousie University [email protected] Interpreting an Abnormal CBC – April 11, 2018 1 Disclosures • No conflicts of interest particularly relevant to this talk • Advisory Boards • Celgene • Co-/sub-investigator on a number therapeutic trials for various hematologic malignancies including leukemia, lymphoma, myeloma • Not discussing “off-label” medication use Interpreting an Abnormal CBC – April 11, 2018 2 Lecture Objectives • 1. Understand normal blood cell production at a very broad level • 2. Develop an initial approach to working up CBC abnormalities • Cytopenias • “-Cytosis” • 3. Recognize concerning complete blood count abnormalities • Recognize hematologic emergencies/red flags • When to refer and our local triage criteria Interpreting an Abnormal CBC – April 11, 2018 3 Refresher- Normal Hematopoiesis Bone marrow Normal bone marrow Interpreting an Abnormal CBC – April 11, 2018 4 Refresher- Normal Hematopoiesis Courtesy of Dr. Clinton Campbell, Hematopathology Interpreting an Abnormal CBC – April 11, 2018 5 Refresher- Normal Hematopoiesis Courtesy of Dr. Clinton Campbell, Hematopathology Interpreting an Abnormal CBC – April 11, 2018 6 Refresher- The Peripheral Blood Smear Courtesy of Dr. Clinton Campbell, Hematopathology Interpreting an Abnormal CBC – April 11, 2018 7 Refresher – The bone marrow Normal bone marrow Interpreting an Abnormal CBC – April 11, 2018 8 Refresher- CBC Parameters Parameter Normal Range* Units WBC