Advanced Blood Cell Identification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reactive Plasmacytosis in a Patient of Acute Myeloid Leukemia at Initial
Open Journal of Clinical & Medical Volume 7 (2021) Case Reports Issue 01 ISSN: 2379-1039 Reactive plasmacytosis in a patient of acute myeloid leukemia at initial presentation – A rare occurrence Sundas Ali; Shahzad Ali Jiskani*; Samina Tufail Amanat; Aliena Sohail *Corresponding Author: Shahzad Ali Jiskani Department of Pathology, Pakistan Institute of Medical Sciences, Islamabad. Email: [email protected] Abstract An increased proliferation of plasma cells has been seen very rarely in patients of Acute Myeloid Leukemia (AML) at the time of initial presentation. In this report, we describe a 64-year old case of AML with reactive plasmacytosis at the time of diagnosis. It has been suggested that paraneoplastic IL-6 production by leuke- mic blasts may be responsible for growth stimulation of plasma cells in marrow. Keywords Acute myeloid leukemia; reactive plasmacytosis; paraneoplastic IL-6. Background The presence of reactive plasmacytosis in patients with acute myeloid leukemia has been shown after chemotherapy. However, it is a rare occurrence in a newly diagnosed AML case. Our patient presen- ted with a moderate proliferation of plasma cells in the bone marrow, along with morphological features consistent with the diagnosis of acute myeloid leukemia with maturation. A careful investigative approach is required to resolve this diagnostic dilemma [1,2]. Case presentation This 64-year old male patient presented to our hospital with complaints of fever, productive cough, generalized weakness and undocumented weight loss for the last one month, and epistaxis and hematuria for the last 5 days. He was a known case of HCV taking no treatment. Physical examination revealed only pallor and mild jaundice. -

Auer Rod-Like Inclusions and Hemophagocytosis in Neoplastic Cells of Multiple Myeloma
Hematology & Transfusion International Journal Case Report Open Access Auer rod-like inclusions and hemophagocytosis in neoplastic cells of multiple myeloma Abstract Volume 1 Issue 3 - 2015 Objective: Several intracytoplasmic morphological changes in the plasma cells of Juan Zhang,1 YanxinLi,1 Shunjun Li,1 Xianyong multiple myeloma have been described previously. However, Auer rod-like inclusions Jiang,2 Yucheng Meng,3 Mingyong Li,1 Yuan and hemophagocytosis are rarely found in these types of cells. In this paper, we intend 1 1 to report a rare case of multiple myeloma. He, Wenfang Huang 1Clinical Laboratory of Sichuan Academy of Medical Science & Methods: Bone marrow aspiration from the right superior iliac spine was examined Sichuan Provincial People’s Hospital, Chengdu, China twice. Cells were stained with May–Grünwald–Giemsa method. Bone scan 2Haematology bone marrow inspection laboratory of Peking demonstrated a focal lesion in the left iliac crest, which was confirmed subsequently Union Medical College Hospital, Beijing, China as a lytic lesion on CT scanning. By flow cytometry, plasma cells expressed CD38, 3Clinical Laboratory of Langfang Hospital of Traditional Chinese CD138, and CD56, CD184 and were negative for CD10, CD19, CD20, CD22, CD27 Medicine, Hebei, China and CyclinD1, with extensive strong Kappa light chain immunostaining. A complete Yanxin Li, Clinical Laboratory of Sichuan blood count and serum chemistry were also examined. Correspondence: Academy of Medical Science & Sichuan provincial People’s Results: It was -
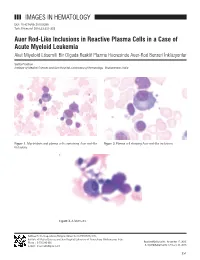
IMAGES in HEMATOLOGY Auer Rod-Like Inclusions in Reactive
IMAGES IN HEMATOLOGY DOI: 10.4274/tjh.2015.0399 Turk J Hematol 2016;33:351-352 Auer Rod-Like Inclusions in Reactive Plasma Cells in a Case of Acute Myeloid Leukemia Akut Miyeloid Lösemili Bir Olguda Reaktif Plazma Hücresinde Auer-Rod Benzeri İnklüzyonlar Sarita Pradhan Institute of Medical Sciences and Sum Hospital, Laboratory of Hematology, Bhubaneswar, India Figure 1. Myeloblasts and plasma cells containing Auer rod-like Figure 2. Plasma cell showing Auer rod-like inclusions. inclusions. Figure 3. A Mott cell. Address for Correspondence/Yazışma Adresi: Sarita PRADHAN, M.D., Institute of Medical Sciences and Sum Hospital, Laboratory of Hematology, Bhubaneswar, India Phone : 9 776 243 866 Received/Geliş tarihi: November 17, 2015 E-mail : [email protected] Accepted/Kabul tarihi: February 23, 2016 351 Pradhan S: Auer Rod-Like Inclusions in Plasma Cells Turk J Hematol 2016;33:351-352 A 61-year-old female presented with decreasing hemoglobin for Keywords: Auer rods, Acute myeloid leukemia, Plasma cells the past 6 months. She had a history of multiple transfusions in the recent past. Laboratory investigations showed hemoglobin Anahtar Sözcükler: Auer cismi, Akut miyeloid lösemi, Plazma of 8.6 g/dL, total blood leukocyte count of 1.13x109/L, and hücreleri platelets of 80x109/L with the presence of occasional circulating blasts. Bone marrow examination revealed the presence of Conflict of Interest: The author of this paper has no conflicts 63% myeloblasts with prominent Auer rods and mild reactive of interest, including specific financial interests, relationships, plasmacytosis (6%). Some of the plasma cells showed Auer and/or affiliations relevant to the subject matter or materials rod-like thin slender inclusions (Figures 1, 2, and 3). -

10 11 Cyto Slides 81-85
NEW YORK STATE CYTOHEMATOLOGY PROFICIENCY TESTING PROGRAM Glass Slide Critique ~ November 2010 Slide 081 Diagnosis: MDS to AML 9 WBC 51.0 x 10 /L 12 Available data: RBC 3.39 x 10 /L 72 year-old female Hemoglobin 9.6 g/dL Hematocrit 29.1 % MCV 86.0 fL Platelet count 16 x 109 /L The significant finding in this case of Acute Myelogenous Leukemia (AML) was the presence of many blast forms. The participant median for blasts, all types was 88. The blast cells in this case (Image 081) are large, irregular in shape and contain large prominent nucleoli. It is difficult to identify a blast cell as a myeloblast without the presence of an Auer rod in the cytoplasm. Auer rods were reported by three participants. Two systems are used to classify AML into subtypes, the French- American-British (FAB) and the World Health Organization (WHO). Most are familiar with the FAB classification. The WHO classification system takes into consideration prognostic factors in classifying AML. These factors include cytogenetic test results, patient’s age, white blood cell count, pre-existing blood disorders and a history of treatment with chemotherapy and/or radiation therapy for a prior cancer. The platelet count in this case was 16,000. Reduced number of platelets was correctly reported by 346 (94%) of participants. Approximately eight percent of participants commented that the red blood cells in this case were difficult to evaluate due to the presence of a bluish hue around the red blood cells. Comments received included, “On slide 081 the morphology was difficult to evaluate since there was a large amount of protein surrounding RBC’s”, “Slide 081 unable to distinguish red cell morphology due to protein” and “Unable to adequately assess morphology on slide 081 due to poor stain”. -
Blood Lab First Week
Blood Lab First Week This is a self propelled powerpoint study atlas of blood cells encountered in examination of the peripheral blood smear. It is a requirement of this course that you begin review of these slides with the first day of class in order to familiarize yourself with terms used in describing peripheral blood morphology. Laboratory final exam questions are derived directly from these slides. The content of these slides may duplicate slides (1-37) listed on the Hematopathology Website. You must familiarize yourself with both sets of slides in order to have the best learning opportunity. Before Beginning This Slide Review • Please read the Laboratory information PDF under Laboratory Resources file to learn about – Preparation of a blood smear – How to select an area of the blood smear for review of morphology and how to perform a white blood cell differential – Platelet estimation – RBC morphology descriptors • Anisocytosis • Poikilocytosis • Hypochromia • Polychromatophilia Normal blood smear. Red blood cells display normal orange pink cytoplasm with central pallor 1/3-1/2 the diameter of the red cell. There is mild variation in size (anisocytosis) but no real variation in shape (poikilocytosis). To the left is a lymphocyte. To the right is a typical neutrophil with the usual 3 segmentations of the nucleus. Med. Utah pathology Normal blood: thin area Ref 2 Normal peripheral blood smear. This field is good for exam of cell morphology, although there are a few minor areas of overlap of red cells. Note that most cells are well dispersed and the normal red blood cell central pallor is noted throughout the smear. -

ORIGINAL ARTICLE PML–Rara Initiates Leukemia by Conferring Properties of Self-Renewal to Committed Promyelocytic Progenitors
Leukemia (2009) 23, 1462–1471 & 2009 Macmillan Publishers Limited All rights reserved 0887-6924/09 $32.00 www.nature.com/leu ORIGINAL ARTICLE PML–RARa initiates leukemia by conferring properties of self-renewal to committed promyelocytic progenitors S Wojiski1,2, FC Guibal3, T Kindler1,2, BH Lee1,2, JL Jesneck4,5, A Fabian1,2, DG Tenen3,6 and DG Gilliland1,2,6 1Division of Hematology, Brigham and Women’s Hospital, Boston, MA, USA; 2Department of Genetics, Howard Hughes Medical Institute, Harvard Medical School, Boston, MA, USA; 3Department of Hematology/Oncology, Harvard Institutes of Medicine, Boston, MA, USA; 4Cancer Program, Broad Institute of Harvard and MIT, Cambridge, MA, USA; 5Department of Pediatric Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA and 6Harvard Stem Cell Institute, Harvard University, Boston, MA, USA Acute promyelocytic leukemia (APL) is characterized by the leukemia stem cells (LSCs) are rare cells contained within hyperproliferation of promyelocytes, progenitors that are the more primitive CD34 þ CD38À population, resembling committed to terminal differentiation into granulocytes, making it an ideal disease in which to study the transforming potential transformed hematopoietic stem cells (HSCs). LSC activity was of less primitive cell types. We utilized a murine model of APL in shown by these investigators in all AML subtypes tested, but which the PML–RARa oncogene is expressed from the were not shown in acute promyelocytic leukemia (APL) endogenous cathepsin G promoter to test the hypothesis that associated with the expression of the PML–RARa fusion as a leukemia stem cell (LSC) activity resides within the differen- consequence of an acquired t(15;17). -

Electron Microscopic and Peroxidase Cytochemical Analysis of Pink Pseudo-Chediak-Higashi Granules in Acute Myelogenous Leukemia1
[CANCER RESEARCH 40, 4473-4481, December 1980] 0008-5472/80/0040-4473S02.00 Electron Microscopic and Peroxidase Cytochemical Analysis of Pink Pseudo-Chediak-Higashi Granules in Acute Myelogenous Leukemia1 William A. Dittman, Robert J. Kramer, and Dorothy F. Bainton2 Sacred Heart Medical Center, Spokane. Washington 99024 [W. A. D.]; Station 5. Pasco. Washington 99301 ¡R.J. K.J; and Department of Pathology. University of California School of Medicine. San Francisco. California 94143 ¡D.F. B.¡ ABSTRACT We have had the opportunity to study 3 patients with AML whose blasts, promyelocytes, and myelocytes contained enor Giant round pink inclusions (=2 jam)were seen in neutrophilic mous round pink inclusions, initially thought to be ingested myeloblasts, promyelocytes, and myelocytes from three pa RBC. Analysis by electron microscopy and peroxidase cyto tients with acute myelogenous leukemia. On preliminary ex chemistry revealed that these pink structures were large per amination of the bone marrow smears, these inclusions looked oxidase-positive granules and therefore also represented ab like ingested red blood cells in that they were pink and not normal variants of the azurophilic (primary) granule population. azurophilic. The bone marrow specimens were processed for In addition, these 3 cases did not have DIC. the electron microscopic demonstration of peroxidase with 3,3'-diaminobenzidine and H2C>2at pH 7.6. In all three cases, the inclusions were determined to be large peroxidase-positive MATERIALS AND METHODS granules since they were limited by a single unit membrane Case Studies and, unlike endocytized red blood cells, were not contained within phagocytic vacuoles. The granules were homogeneously Case 1. -

Difference Between Promyelocyte and Myelocyte Key Difference
Difference Between Promyelocyte and Myelocyte www.differencebetween.com Key Difference - Promyelocyte vs Myelocyte Granulated blood cells include eosinophils, basophils, and neutrophils which participate in a variety of functions in the body. The precursor stem cells of these cells which arise from the hematopoietic stem cells are of the myeloid lineage. Myeloblasts are the precursor cells of granulated blood cells. Myeloblasts then mature into promyelocytes, myelocytes, metamyelocytes, bands, and segments to finally give rise to granulocytes in the peripheral blood tissue. The development process is known as Granulopoiesis. Promyelocyte is the second stage of Myeloblast development. Myelocyte is the third stage of Myeloblast development. The key difference between the promyelocyte and the myelocyte is the level of differentiation it exhibits. Promyelocytes do not show differentiation while myelocytes show differentiation. What is a Promyelocyte? Promyelocyte is the second stage of the Myeloblast development process. The promyelocyte is larger than the myeloblast. It has a diameter of 12-25µm and is the largest cell type in the myeloid series. It has a prominent nucleus, and the nucleus is placed slightly intended in the cytoplasm. Chromatin and nucleoli are prominent in this. Final structures of chromatin can be identified through microscopic observations. Towards the complete maturation of the promyelocyte, the chromatins appear as well-condensed structures. The condensed chromatin is placed along the nuclear membrane. The cytoplasm of the promyelocyte is granulated, and these granules are termed as the primary azurophilic granules. Since the promyelocyte is not differentiated, it is formed of a basophilic cytoplasm. The cell organelle organization is prominent in the promyelocyte stage of the blood cells. -

Henry Ford Hospital Clinicopathological Conference: Metamorphosis in Chronic Granulocytic Leukemia in a 60-Year-Old Man
Henry Ford Hospital Medical Journal Volume 28 Number 1 Article 7 3-1980 Henry Ford Hospital Clinicopathological Conference: Metamorphosis in chronic granulocytic leukemia in a 60-year-old man Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation (1980) "Henry Ford Hospital Clinicopathological Conference: Metamorphosis in chronic granulocytic leukemia in a 60-year-old man," Henry Ford Hospital Medical Journal : Vol. 28 : No. 1 , 37-46. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol28/iss1/7 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. Henry Ford Hosp Med Journal Vol 28, No 1, 1980 Henry Ford Hospital Clinicopathological Conference Metamorphosis in chronic granulocytic leukemia in a 60-year-old man Participants: Protocol: Dr. Ellis J. Van Slyck, Department of Internal Medicine, Division of Hematology Discussant: Dr. Robert K. Nixon, Department of Internal Medicine, Second Medical Division Radiology: Dr. Mark C. Weingarden, Department of Diagnostic Radiology Pathology: Dr. John W. Rebuck, Department of Pathology, Division of Hematology Case Presentation As an outpatient, his myleran dosage was gradually tapered as the This 60-year-old white man came to Henry Ford Hospital in blood counts responded. On February 27, the white count was December 1975, complaining of increased fatigability over the 40,000 and the platelet count 400,000. On May 10, the white preceding year. -

Perforin-2 Is Essential for Intracellular Defense of Parenchymal Cells And
RESEARCH ARTICLE elifesciences.org Perforin-2 is essential for intracellular defense of parenchymal cells and phagocytes against pathogenic bacteria Ryan M McCormack1, Lesley R de Armas1, Motoaki Shiratsuchi1, Desiree G Fiorentino1, Melissa L Olsson1, Mathias G Lichtenheld1, Alejo Morales1, Kirill Lyapichev1, Louis E Gonzalez1, Natasa Strbo1, Neelima Sukumar2, Olivera Stojadinovic3, Gregory V Plano1, George P Munson1, Marjana Tomic- Canic3, Robert S Kirsner3, David G Russell2, Eckhard R Podack1* 1Department of Microbiology and Immunology, Miller School of Medicine, University of Miami, Miami, United States; 2Department of Microbiology and Immunology, College of Veterinary Medicine, Cornell University, Ithaca, United States; 3Wound Healing and Regenerative Medicine Research Program, Department of Dermatology and Cutaneous Surgery, Miller School of Medicine, University of Miami, Miami, United States Abstract Perforin-2 (MPEG1) is a pore-forming, antibacterial protein with broad-spectrum activity. Perforin-2 is expressed constitutively in phagocytes and inducibly in parenchymal, tissue-forming cells. In vitro, Perforin-2 prevents the intracellular replication and proliferation of bacterial pathogens in these cells. Perforin-2 knockout mice are unable to control the systemic dissemination of methicillin-resistant Staphylococcus aureus (MRSA) or Salmonella typhimurium and perish shortly after epicutaneous or orogastric infection respectively. In contrast, Perforin-2-sufficient littermates clear the infection. Perforin-2 is a transmembrane protein of cytosolic vesicles -derived from multiple organelles- that translocate to and fuse with bacterium containing vesicles. Subsequently, Perforin-2 polymerizes and forms large clusters of 100 A˚ pores in the bacterial surface with Perforin-2 cleavage products present in bacteria. Perforin-2 is also required for the bactericidal activity of reactive oxygen and nitrogen species and hydrolytic enzymes. -

Bone Marrow Aspirate A Bone Marrow Film Should First Be Examined Macroscopically to Make Sure That Particles Or Fragments Are Present
Aspiration of the BM Satisfactory samples can usually be aspirated from the Sternum Anterior or posterior iliac spines Aspiration from only one site can give rise to misleading information; this is particularly true in aplastic anaemia as the marrow may be affected partially. There is little advantage in aspirating more than 0.3 ml of marrow fluid from a single site for morphological examination as this increases peripheral blood dilution. Bone marrow aspirate A bone marrow film should first be examined macroscopically to make sure that particles or fragments are present. Bone marrow aspirates which lack particles may be diluted with peripheral blood and may therefore be unrepresentative. An ideal bone marrow film with particles is shown. Even films without fragments are worth examining as useful information may be gained. However, assessment of cellularity and megakaryocyte numbers is unreliable and dilution with peripheral blood may lead to lymphocytes and neutrophils being over- represented in the differential count. Erythroid series Myeloid series Megakaryocytic series *Proerythroblast **Early erythroblast ***Intermediate erythroblast ****Late erythroblast Erythroid precursors Normal red cells are produced in the bone marrow from erythroid precursors or erythroblasts. The earliest morphologically recognisable red cell precursor is derived from an erythroid progenitor cell which in turn is derived from a multipotent haemopoietic progenitor cell. proerythroblast Normal proerythroblast [dark red arrow] in the bone marrow. This is a large cell with a round nucleus and a finely stippled chromatin pattern. Nucleoli are sometimes apparent. The cytoplasm is moderately to strongly basophilic. There may be a paler staining area of cytoplasm surrounding the nucleus. Normal erythroblasts in the BM . -

Auer Rod-Like Crystal Inclusions in Plasma Cells of Multiple Myeloma Sung-Hee Oh, Chan Jeoung Park
DOI: 10.5045/kjh.2010.45.4.222 The Korean Journal of Hematology Volume 45ㆍNumber 4ㆍDecember 2010 Auer rod-like crystal inclusions in plasma cells of multiple myeloma Sung-hee Oh, Chan Jeoung Park Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea A 65-year-old man with 10-year history of non-insulin dependent diabetes mellitus presented with anemia and thrombocytopenia, which lasted for 3 months. Blood cell counts were: WBC, 5.1×109/L; hemoglobin, 9.0 g/dL; plate- let, 64×109/L. Peripheral blood film showed no rouleaux formation. Routine chemistry showed: calcium, 9.4 mg/dL; blood urea nitrogen/creatinine, 12/1.1 mg/dL; protein/albumin, 6.3/3.5 g/dL. Radiologic studies showed suspicious os- teolytic lesions on the humerus and distal clavicles. Serum protein electrophoresis revealed M-peak of 0.2 g/dL, and immunofixation electrophoresis showed a zone of restriction in the kappa light chain, suggesting monoclonal component. Urine protein electrophoresis revealed free kappa-type Bence-Jones proteinuria (59.3% of urine protein). Bone marrow aspirates showed many plasma cells (64.0% of nucleated cells) with Auer rod-like crystal inclusions in the cytoplasm. (A) Engulfed Auer rod-like inclusions in numerous histiocytes and free Auer rod-like inclusions in the backgrounds were observed. (B) Bone marrow biopsy showed decreased cellularity with interstitial and nodular infiltration of plasma cells. Immunohistochemical findings confirmed the kappa monoclonality of plasma cells. Despite 2 cycles of dexamethasone treatment without complications, the patient still suffers from Bence-Jones proteinuria and worsened bone pain.