Perforin-2 Is Essential for Intracellular Defense of Parenchymal Cells And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calreticulin—Multifunctional Chaperone in Immunogenic Cell Death: Potential Significance As a Prognostic Biomarker in Ovarian
cells Review Calreticulin—Multifunctional Chaperone in Immunogenic Cell Death: Potential Significance as a Prognostic Biomarker in Ovarian Cancer Patients Michal Kielbik *, Izabela Szulc-Kielbik and Magdalena Klink Institute of Medical Biology, Polish Academy of Sciences, 106 Lodowa Str., 93-232 Lodz, Poland; [email protected] (I.S.-K.); [email protected] (M.K.) * Correspondence: [email protected]; Tel.: +48-42-27-23-636 Abstract: Immunogenic cell death (ICD) is a type of death, which has the hallmarks of necroptosis and apoptosis, and is best characterized in malignant diseases. Chemotherapeutics, radiotherapy and photodynamic therapy induce intracellular stress response pathways in tumor cells, leading to a secretion of various factors belonging to a family of damage-associated molecular patterns molecules, capable of inducing the adaptive immune response. One of them is calreticulin (CRT), an endoplasmic reticulum-associated chaperone. Its presence on the surface of dying tumor cells serves as an “eat me” signal for antigen presenting cells (APC). Engulfment of tumor cells by APCs results in the presentation of tumor’s antigens to cytotoxic T-cells and production of cytokines/chemokines, which activate immune cells responsible for tumor cells killing. Thus, the development of ICD and the expression of CRT can help standard therapy to eradicate tumor cells. Here, we review the physiological functions of CRT and its involvement in the ICD appearance in malignant dis- ease. Moreover, we also focus on the ability of various anti-cancer drugs to induce expression of surface CRT on ovarian cancer cells. The second aim of this work is to discuss and summarize the prognostic/predictive value of CRT in ovarian cancer patients. -

Apoptosis by Incomplete Infection
rESEArcH HIGHLIGHtS Apoptosis by incomplete infection Constraining inflammation κ Infection with human immunodeficiency virus (HIV) is The transcription factor NF- B is a critical mediator of Toll-like recep- characterized by progressive apoptosis of CD4+ T cells, and tor (TLR) signaling in response to a range of activators. In Genes & although some of the molecular participants in this process are Development, Barish et al. provide genetic and genomic evidence that known, the precise details remain unclear. Greene and colleagues the transcription factor Bcl-6 broadly antagonizes the TLR–NF-κB in Cell report the unexpected finding that nonproductive infection network. Genome-wide expression analyses and chromatin-immuno- of CD4+ T cells can also result in apoptosis. The authors use a precipitation sequencing (ChIP-Seq) show that Bcl-6 is a basal- and human lymphoid aggregation culture system to recapitulate in lipopolysaccharide (LPS)-induced inhibitor of many inflammatory vitro the events of HIV infection in the lymph node. The addition modules in bone marrow–derived macrophages. Bcl-6 controls one of HIV to this culture system results in the apoptosis of not only third of the LPS-elicited transcriptome, and sites for Bcl-6 and the productively infected but also nonpermissive CD4+ cells. Apoptosis NF-κB subunit p65 are located together in a nucleosomal window of the latter requires fusion with HIV and the initiation but not (200 base pairs) in 25% of the gene network controlled by TLR–NF-κB. completion of viral reverse transcription. The accumulation Stimulation of TLR4 reciprocally diminishes or enhances the binding of of incomplete HIV reverse transcripts results in activation of Bcl-6 and p65 to enhancer regions at target genes encoding key inflam- caspase-1 and caspase-3 and release of the inflammatory cytokine matory molecules, such as IL-1 and various chemokines. -

Mechanism of Mammalian Cell Lysis Mediated by Peptide Defensins
Mechanism of Mammalian Cell Lysis Mediated by Peptide Defensins Evidence for an Initial Alteration of the Plasma Membrane Alan Lichtenstein Division ofHematology/Oncology, Department ofMedicine, Veterans Administration Wadsworth-UCLA Medical Center, Los Angeles, California 90073 Abstract targets, are three small (mol wt 3,900) cationic peptides termed defensins or human neutrophil peptides 1, 2, and 3 (HNP Defensins induce ion channels in model lipid bilayers and per- 1-3).' The human defensins are secreted by activated PMNs meabilize the membranes of Escherichia coli. We investigated (10) and could, therefore, function as cytotoxins during anti- whether similar membrane-active events occur during defensin- body-dependent cellular cytotoxicity. In addition, a synergistic mediated cytolysis of tumor cells. Although defensin-treated cytotoxic effect occurs when target cells are exposed to a combi- K562 targets did not release chromium-labeled cytoplasmic nation of defensins and toxic oxidants (8). It is, thus, conceiv- components for 5-6 h, they experienced a rapid collapse able that defensins may play a role in tissue injury seen during (within minutes) of the membrane potential, efflux of rubidium, inflammatory or infectious processes. and influx of trypan blue. Defensin treatment also blunted the To investigate the mechanism of defensin-induced injury, subsequent acidification response induced by nigericin, thereby we have utilized continuously cultured tumor cells as model further supporting the notion of enhanced transmembrane ion targets. In previous studies (11, 12), K562 cells exposed to de- flow during exposure. These initial effects on the plasma mem- fensins began to release radiolabeled chromium after a lag pe- brane were not sufficient for subsequent lysis; a second phase of riod of 3-4 h. -
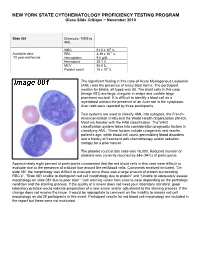
10 11 Cyto Slides 81-85
NEW YORK STATE CYTOHEMATOLOGY PROFICIENCY TESTING PROGRAM Glass Slide Critique ~ November 2010 Slide 081 Diagnosis: MDS to AML 9 WBC 51.0 x 10 /L 12 Available data: RBC 3.39 x 10 /L 72 year-old female Hemoglobin 9.6 g/dL Hematocrit 29.1 % MCV 86.0 fL Platelet count 16 x 109 /L The significant finding in this case of Acute Myelogenous Leukemia (AML) was the presence of many blast forms. The participant median for blasts, all types was 88. The blast cells in this case (Image 081) are large, irregular in shape and contain large prominent nucleoli. It is difficult to identify a blast cell as a myeloblast without the presence of an Auer rod in the cytoplasm. Auer rods were reported by three participants. Two systems are used to classify AML into subtypes, the French- American-British (FAB) and the World Health Organization (WHO). Most are familiar with the FAB classification. The WHO classification system takes into consideration prognostic factors in classifying AML. These factors include cytogenetic test results, patient’s age, white blood cell count, pre-existing blood disorders and a history of treatment with chemotherapy and/or radiation therapy for a prior cancer. The platelet count in this case was 16,000. Reduced number of platelets was correctly reported by 346 (94%) of participants. Approximately eight percent of participants commented that the red blood cells in this case were difficult to evaluate due to the presence of a bluish hue around the red blood cells. Comments received included, “On slide 081 the morphology was difficult to evaluate since there was a large amount of protein surrounding RBC’s”, “Slide 081 unable to distinguish red cell morphology due to protein” and “Unable to adequately assess morphology on slide 081 due to poor stain”. -

I M M U N O L O G Y Core Notes
II MM MM UU NN OO LL OO GG YY CCOORREE NNOOTTEESS MEDICAL IMMUNOLOGY 544 FALL 2011 Dr. George A. Gutman SCHOOL OF MEDICINE UNIVERSITY OF CALIFORNIA, IRVINE (Copyright) 2011 Regents of the University of California TABLE OF CONTENTS CHAPTER 1 INTRODUCTION...................................................................................... 3 CHAPTER 2 ANTIGEN/ANTIBODY INTERACTIONS ..............................................9 CHAPTER 3 ANTIBODY STRUCTURE I..................................................................17 CHAPTER 4 ANTIBODY STRUCTURE II.................................................................23 CHAPTER 5 COMPLEMENT...................................................................................... 33 CHAPTER 6 ANTIBODY GENETICS, ISOTYPES, ALLOTYPES, IDIOTYPES.....45 CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION..................................................................53 CHAPTER 8 GENETIC BASIS OF ANTIBODY DIVERSITY...................................61 CHAPTER 9 IMMUNOGLOBULIN BIOSYNTHESIS ...............................................69 CHAPTER 10 BLOOD GROUPS: ABO AND Rh .........................................................77 CHAPTER 11 CELL-MEDIATED IMMUNITY AND MHC ........................................83 CHAPTER 12 CELL INTERACTIONS IN CELL MEDIATED IMMUNITY ..............91 CHAPTER 13 T-CELL/B-CELL COOPERATION IN HUMORAL IMMUNITY......105 CHAPTER 14 CELL SURFACE MARKERS OF T-CELLS, B-CELLS AND MACROPHAGES...............................................................111 -

1. Introduction to Immunology Professor Charles Bangham ([email protected])
MCD Immunology Alexandra Burke-Smith 1. Introduction to Immunology Professor Charles Bangham ([email protected]) 1. Explain the importance of immunology for human health. The immune system What happens when it goes wrong? persistent or fatal infections allergy autoimmune disease transplant rejection What is it for? To identify and eliminate harmful “non-self” microorganisms and harmful substances such as toxins, by distinguishing ‘self’ from ‘non-self’ proteins or by identifying ‘danger’ signals (e.g. from inflammation) The immune system has to strike a balance between clearing the pathogen and causing accidental damage to the host (immunopathology). Basic Principles The innate immune system works rapidly (within minutes) and has broad specificity The adaptive immune system takes longer (days) and has exisite specificity Generation Times and Evolution Bacteria- minutes Viruses- hours Host- years The pathogen replicates and hence evolves millions of times faster than the host, therefore the host relies on a flexible and rapid immune response Out most polymorphic (variable) genes, such as HLA and KIR, are those that control the immune system, and these have been selected for by infectious diseases 2. Outline the basic principles of immune responses and the timescales in which they occur. IFN: Interferon (innate immunity) NK: Natural Killer cells (innate immunity) CTL: Cytotoxic T lymphocytes (acquired immunity) 1 MCD Immunology Alexandra Burke-Smith Innate Immunity Acquired immunity Depends of pre-formed cells and molecules Depends on clonal selection, i.e. growth of T/B cells, release of antibodies selected for antigen specifity Fast (starts in mins/hrs) Slow (starts in days) Limited specifity- pathogen associated, i.e. -

Which Is Directly Mediated by Fumigatus Aspergillus Antifungal Activity Against Human NK Cells Display Importa
Human NK Cells Display Important Antifungal Activity against Aspergillus fumigatus , Which Is Directly Mediated by IFN- γ Release This information is current as of September 28, 2021. Maria Bouzani, Michael Ok, Allison McCormick, Frank Ebel, Oliver Kurzai, C. Oliver Morton, Hermann Einsele and Juergen Loeffler J Immunol 2011; 187:1369-1376; Prepublished online 22 June 2011; Downloaded from doi: 10.4049/jimmunol.1003593 http://www.jimmunol.org/content/187/3/1369 http://www.jimmunol.org/ Supplementary http://www.jimmunol.org/content/suppl/2011/06/22/jimmunol.100359 Material 3.DC1 References This article cites 41 articles, 14 of which you can access for free at: http://www.jimmunol.org/content/187/3/1369.full#ref-list-1 Why The JI? Submit online. by guest on September 28, 2021 • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2011 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Human NK Cells Display Important Antifungal Activity against Aspergillus fumigatus, Which Is Directly Mediated by IFN-g Release Maria Bouzani,* Michael Ok,* Allison McCormick,† Frank Ebel,† Oliver Kurzai,‡,x C. -
Blood Lab First Week
Blood Lab First Week This is a self propelled powerpoint study atlas of blood cells encountered in examination of the peripheral blood smear. It is a requirement of this course that you begin review of these slides with the first day of class in order to familiarize yourself with terms used in describing peripheral blood morphology. Laboratory final exam questions are derived directly from these slides. The content of these slides may duplicate slides (1-37) listed on the Hematopathology Website. You must familiarize yourself with both sets of slides in order to have the best learning opportunity. Before Beginning This Slide Review • Please read the Laboratory information PDF under Laboratory Resources file to learn about – Preparation of a blood smear – How to select an area of the blood smear for review of morphology and how to perform a white blood cell differential – Platelet estimation – RBC morphology descriptors • Anisocytosis • Poikilocytosis • Hypochromia • Polychromatophilia Normal blood smear. Red blood cells display normal orange pink cytoplasm with central pallor 1/3-1/2 the diameter of the red cell. There is mild variation in size (anisocytosis) but no real variation in shape (poikilocytosis). To the left is a lymphocyte. To the right is a typical neutrophil with the usual 3 segmentations of the nucleus. Med. Utah pathology Normal blood: thin area Ref 2 Normal peripheral blood smear. This field is good for exam of cell morphology, although there are a few minor areas of overlap of red cells. Note that most cells are well dispersed and the normal red blood cell central pallor is noted throughout the smear. -

ORIGINAL ARTICLE PML–Rara Initiates Leukemia by Conferring Properties of Self-Renewal to Committed Promyelocytic Progenitors
Leukemia (2009) 23, 1462–1471 & 2009 Macmillan Publishers Limited All rights reserved 0887-6924/09 $32.00 www.nature.com/leu ORIGINAL ARTICLE PML–RARa initiates leukemia by conferring properties of self-renewal to committed promyelocytic progenitors S Wojiski1,2, FC Guibal3, T Kindler1,2, BH Lee1,2, JL Jesneck4,5, A Fabian1,2, DG Tenen3,6 and DG Gilliland1,2,6 1Division of Hematology, Brigham and Women’s Hospital, Boston, MA, USA; 2Department of Genetics, Howard Hughes Medical Institute, Harvard Medical School, Boston, MA, USA; 3Department of Hematology/Oncology, Harvard Institutes of Medicine, Boston, MA, USA; 4Cancer Program, Broad Institute of Harvard and MIT, Cambridge, MA, USA; 5Department of Pediatric Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA and 6Harvard Stem Cell Institute, Harvard University, Boston, MA, USA Acute promyelocytic leukemia (APL) is characterized by the leukemia stem cells (LSCs) are rare cells contained within hyperproliferation of promyelocytes, progenitors that are the more primitive CD34 þ CD38À population, resembling committed to terminal differentiation into granulocytes, making it an ideal disease in which to study the transforming potential transformed hematopoietic stem cells (HSCs). LSC activity was of less primitive cell types. We utilized a murine model of APL in shown by these investigators in all AML subtypes tested, but which the PML–RARa oncogene is expressed from the were not shown in acute promyelocytic leukemia (APL) endogenous cathepsin G promoter to test the hypothesis that associated with the expression of the PML–RARa fusion as a leukemia stem cell (LSC) activity resides within the differen- consequence of an acquired t(15;17). -

The Host Defense Peptide Cathelicidin Is Required for NK Cell-Mediated Suppression of Tumor Growth
The Host Defense Peptide Cathelicidin Is Required for NK Cell-Mediated Suppression of Tumor Growth This information is current as Amanda S. Büchau, Shin Morizane, Janet Trowbridge, of September 27, 2021. Jürgen Schauber, Paul Kotol, Jack D. Bui and Richard L. Gallo J Immunol 2010; 184:369-378; Prepublished online 30 November 2009; doi: 10.4049/jimmunol.0902110 Downloaded from http://www.jimmunol.org/content/184/1/369 References This article cites 68 articles, 24 of which you can access for free at: http://www.jimmunol.org/content/184/1/369.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 27, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2010 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology The Host Defense Peptide Cathelicidin Is Required for NK Cell-Mediated Suppression of Tumor Growth Amanda S. Bu¨chau,*,† Shin Morizane,*,† Janet Trowbridge,*,†,1 Ju¨rgen Schauber,*,†,‡ Paul Kotol,*,† Jack D. -

Difference Between Promyelocyte and Myelocyte Key Difference
Difference Between Promyelocyte and Myelocyte www.differencebetween.com Key Difference - Promyelocyte vs Myelocyte Granulated blood cells include eosinophils, basophils, and neutrophils which participate in a variety of functions in the body. The precursor stem cells of these cells which arise from the hematopoietic stem cells are of the myeloid lineage. Myeloblasts are the precursor cells of granulated blood cells. Myeloblasts then mature into promyelocytes, myelocytes, metamyelocytes, bands, and segments to finally give rise to granulocytes in the peripheral blood tissue. The development process is known as Granulopoiesis. Promyelocyte is the second stage of Myeloblast development. Myelocyte is the third stage of Myeloblast development. The key difference between the promyelocyte and the myelocyte is the level of differentiation it exhibits. Promyelocytes do not show differentiation while myelocytes show differentiation. What is a Promyelocyte? Promyelocyte is the second stage of the Myeloblast development process. The promyelocyte is larger than the myeloblast. It has a diameter of 12-25µm and is the largest cell type in the myeloid series. It has a prominent nucleus, and the nucleus is placed slightly intended in the cytoplasm. Chromatin and nucleoli are prominent in this. Final structures of chromatin can be identified through microscopic observations. Towards the complete maturation of the promyelocyte, the chromatins appear as well-condensed structures. The condensed chromatin is placed along the nuclear membrane. The cytoplasm of the promyelocyte is granulated, and these granules are termed as the primary azurophilic granules. Since the promyelocyte is not differentiated, it is formed of a basophilic cytoplasm. The cell organelle organization is prominent in the promyelocyte stage of the blood cells. -

Advanced Blood Cell Identification
ADVANCED BLOOD CELL ID: LEUKOCYTES AND ERYTHROCYTES IN AN ACUTE LEUKEMIA Educational commentary is provided for participants enrolled in program #259- Advanced Blood Cell Identification. This virtual blood cell identification program includes case studies with more difficult challenges. To view the blood cell images in more detail, click on the sample identification numbers underlined in the paragraphs below. This will open a virtual image of the selected cell and the surrounding fields. If the image opens in the same window as the commentary, saving the commentary PDF and opening it outside your browser will allow you to switch between the commentary and the images more easily. Click on this link for the API ImageViewerTM Instructions. Learning Outcomes After completing this exercise, participants should be able to: • Discuss morphologic characteristics of normal peripheral blood leukocytes. • Describe morphologic features of immature granulocytes. • Identify morphologic abnormalities in erythrocyte shape and chromaticity/coloration. Case Study An 18 year old male was seen by his physician for bruising and severe nosebleeds. His CBC results are as follows: WBC=7.5 x 109/L, RBC=2.79 1012/L, Hgb=8.7 g/dL, Hct=24.6%, MCV=88 fL, MCH=31 pg, MCHC=35 g/dL, RDW=18.4%, Platelet=41 x 109/L, MPV=10.4. Educational Commentary The cells selected for identification and discussion in this exercise are from the peripheral blood smear of an 18 year old man diagnosed with acute promyelocytic leukemia (APL). APL is also referred to as acute myeloid leukemia, M3 (AML-M3). As with other acute leukemias, APL has a rapid onset.