Seromic Profiling of Ovarian and Pancreatic Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cancer Immunity (1 December 2006) Vol
Cancer Immun 1424-9634Academy of Cancer Immunology Cancer Immunity (1 December 2006) Vol. 6, p. 12 Submitted: 26 September 2006. Accepted: 10 October 2006. Copyright © 2006 by Andrew J. G. Simpson 061012 Article Physical interaction of two cancer-testis antigens, MAGE-C1 (CT7) and NY-ESO-1 (CT6) Hearn J. Cho1*,**, Otavia L. Caballero2*, Sacha Gnjatic2, Valéria C. C. Andrade3, Gisele W. Colleoni3, Andre L. Vettore4, Hasina H. Outtz1, Sheila Fortunato2, Nasser Altorki1, Cathy A. Ferrera1, Ramon Chua2, Achim A. Jungbluth2, Yao-Tseng Chen1, Lloyd J. Old2 and Andrew J. G. Simpson2 1Weill Medical College of Cornell University, 1300 York Avenue, New York, NY 10021, USA 2Ludwig Institute for Cancer Research, New York Branch at Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021, USA 3Escola Paulista de Medicina, Universidade Federal de Sao Paulo, Sao Paulo, SP, Brazil 4Ludwig Institute for Cancer Research, Sao Paulo Branch, Sao Paulo, SP, Brazil *These authors contributed equally to this work **Present address: NYU Cancer Institute, New York University School of Medicine, 550 First Avenue, New York, NY 10016, USA Contributed by: LJ Old Cancer/testis (CT) antigens are the protein products of germ line- encoded on the X chromosome (CT-X antigens) and those that associated genes that are activated in a wide variety of tumors and can are not (non-X CT antigens) (1). elicit autologous cellular and humoral immune responses. CT It is estimated that 10% of the genes on the X-chromosome antigens can be divided between those that are encoded on the X belong to CT-X families (5). -

Cancer-Testis Antigens MAGEA Proteins Are Incorporated Into Extracellular Vesicles Released by Cells
www.oncotarget.com Oncotarget, 2019, Vol. 10, (No. 38), pp: 3694-3708 Research Paper Cancer-testis antigens MAGEA proteins are incorporated into extracellular vesicles released by cells Anneli Kuldkepp1, Magda Karakai1, Eve Toomsoo1, Olavi Reinsalu1 and Reet Kurg1 1Institute of Technology, University of Tartu, Tartu, Estonia Correspondence to: Reet Kurg, email: [email protected] Keywords: cancer-testis antigens; MAGEA; extracellular vesicles; microvesicles Received: December 13, 2018 Accepted: May 13, 2019 Published: June 04, 2019 Copyright: Kuldkepp et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Melanoma-associated antigen A (MAGEA) family proteins represent a class of tumor antigens that are expressed in a variety of malignant tumors, but their expression in normal tissues is restricted to germ cells. MAGEA family consists of eleven proteins that are highly conserved sharing the common MAGE homology domain (MHD). In the current study, we show that MAGEA4 and MAGEA10 proteins are incorporated into extracellular vesicles released by mouse fibroblast and human osteosarcoma U2OS cells and are expressed, at least partly, on the surface of released EVs. The C-terminal part of the protein containing MHD domain is required for this activity. Expression of MAGEA proteins induced the budding of cells and formation of extracellular vesicles with 150 to 1500 nm in diameter. Our data suggest that the release of MAGEA-positive EVs is at least to some extent induced by the expression of MAGEA proteins itself. -
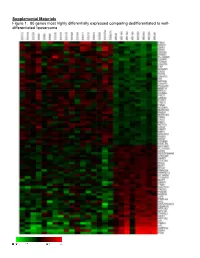
Supplemental Materials Figure 1. 80 Genes Most Highly Differentially Expressed Comparing Dedifferentiated to Well- Differentiated Liposarcoma
Supplemental Materials Figure 1. 80 genes most highly differentially expressed comparing dedifferentiated to well- differentiated liposarcoma Figure 2. Validation of microa CDC2, RACGAP1, FGFR-2, MAD2 and CITED1 200 CDK4 by Liposarcoma Subtype 16 0 12 0 80 Expression40 Level 0 Nl Fat rray data by quantitative RT-P Well Diff 16 0 p16 by Liposarcoma Subtype Dediff 12 0 80 Myxoid Expression40 Level Round Cell 0 12 0 MDM2 by Liposarcoma Subtype 10 0 Pleomorphic Nl Fat 80 60 40 Expression Level 20 RACGAP1 by LiposarcomaWell Diff Subtype 0 70 CR for genes CDK4, MDM2, p16, 60 Dediff 50 Nl Fat 40 30 Myxoid Expression20 Level Well Diff 10 50 0 Round Cell CDC2 by Liposarcoma Subtype Dediff 40 Nl Fat Pleomorphic 30 Myxoid 20 Expression Level Well Diff 10 MAD2L1 by Liposarcoma Subtype Round Cell 60 0 50 Dediff Pleomorphic 40 Nl Fat 30 Myxoid 20 Expression Level Well Diff 10 FGFR-2 by Liposarcoma Subtype 0 Round Cell 200 16 0 Dediff Nl Fat Pleomorphic 12 0 Myxoid 80 Expression Level Well Diff 40 Round Cell 0 Dediff Pleomorphic Nl Fat Myxoid Well Diff CITED1 by Liposarcoma Subtype Round Cell 10 0 80 Dediff Pleomorphic 60 40 Myxoid Expression Level 20 0 Round Cell Nl Fat Pleomorphic Well Diff Dediff Myxoid Round Cell Pleomorphic Table 1. 142 Gene classifier for liposarcoma subtype The genes used for each pair wise subtype comparison are grouped together. The flag column indicates which genes are unique to each subtype comparison. The values show the mean expression levels (actually the mean of the log expression levels was computed and than transformed back to absolute expression level). -

Meta-Analysis of Nasopharyngeal Carcinoma
BMC Genomics BioMed Central Research article Open Access Meta-analysis of nasopharyngeal carcinoma microarray data explores mechanism of EBV-regulated neoplastic transformation Xia Chen†1,2, Shuang Liang†1, WenLing Zheng1,3, ZhiJun Liao1, Tao Shang1 and WenLi Ma*1 Address: 1Institute of Genetic Engineering, Southern Medical University, Guangzhou, PR China, 2Xiangya Pingkuang associated hospital, Pingxiang, Jiangxi, PR China and 3Southern Genomics Research Center, Guangzhou, Guangdong, PR China Email: Xia Chen - [email protected]; Shuang Liang - [email protected]; WenLing Zheng - [email protected]; ZhiJun Liao - [email protected]; Tao Shang - [email protected]; WenLi Ma* - [email protected] * Corresponding author †Equal contributors Published: 7 July 2008 Received: 16 February 2008 Accepted: 7 July 2008 BMC Genomics 2008, 9:322 doi:10.1186/1471-2164-9-322 This article is available from: http://www.biomedcentral.com/1471-2164/9/322 © 2008 Chen et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: Epstein-Barr virus (EBV) presumably plays an important role in the pathogenesis of nasopharyngeal carcinoma (NPC), but the molecular mechanism of EBV-dependent neoplastic transformation is not well understood. The combination of bioinformatics with evidences from biological experiments paved a new way to gain more insights into the molecular mechanism of cancer. Results: We profiled gene expression using a meta-analysis approach. Two sets of meta-genes were obtained. Meta-A genes were identified by finding those commonly activated/deactivated upon EBV infection/reactivation. -

Environmental Influences on Endothelial Gene Expression
ENDOTHELIAL CELL GENE EXPRESSION John Matthew Jeff Herbert Supervisors: Prof. Roy Bicknell and Dr. Victoria Heath PhD thesis University of Birmingham August 2012 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. ABSTRACT Tumour angiogenesis is a vital process in the pathology of tumour development and metastasis. Targeting markers of tumour endothelium provide a means of targeted destruction of a tumours oxygen and nutrient supply via destruction of tumour vasculature, which in turn ultimately leads to beneficial consequences to patients. Although current anti -angiogenic and vascular targeting strategies help patients, more potently in combination with chemo therapy, there is still a need for more tumour endothelial marker discoveries as current treatments have cardiovascular and other side effects. For the first time, the analyses of in-vivo biotinylation of an embryonic system is performed to obtain putative vascular targets. Also for the first time, deep sequencing is applied to freshly isolated tumour and normal endothelial cells from lung, colon and bladder tissues for the identification of pan-vascular-targets. Integration of the proteomic, deep sequencing, public cDNA libraries and microarrays, delivers 5,892 putative vascular targets to the science community. -

Review Article Gas7 Is Required for Mesenchymal Stem Cell-Derived Bone Development
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Crossref Hindawi Publishing Corporation Stem Cells International Volume 2013, Article ID 137010, 6 pages http://dx.doi.org/10.1155/2013/137010 Review Article Gas7 Is Required for Mesenchymal Stem Cell-Derived Bone Development Chuck C.-K. Chao, Feng-Chun Hung, and Jack J. Chao Department of Biochemistry and Molecular Biology and Institute of Biomedical Sciences, College of Medicine, Chang Gung University, Taoyuan 333, Taiwan Correspondence should be addressed to Chuck C.-K. Chao; [email protected] Received 20 January 2013; Accepted 12 May 2013 Academic Editor: Gael¨ Y. Rochefort Copyright © 2013 Chuck C.-K. Chao et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Mesenchymal stem cells (MSCs) can differentiate into osteoblasts and lead to bone formation in the body. Osteoblast differentiation and bone development are regulated by a network of molecular signals and transcription factors induced by several proteins, including BMP2, osterix, and Runx2. We recently observed that the growth-arrest-specific 7 gene (Gas7) is upregulated during differentiation of human MSCs into osteoblasts. Downregulation of Gas7 using short-hairpin RNA decreased the expression of Runx2, a master regulator of osteogenesis, and its target genes (alkaline phosphatase, type I collagen, osteocalcin, and osteopontin). In addition, knockdown of Gas7 decreased the mineralization of dexamethasone-treated MSCs in culture. Conversely, ectopic expression of Gas7 induced Runx2-dependent transcriptional activity and gene expression leading to osteoblast differentiation and matrix mineralization. -

Expression of Cancer-Testis Antigens MAGEA1, MAGEA3, ACRBP, PRAME, SSX2, and CTAG2 in Myxoid and Round Cell Liposarcoma
Modern Pathology (2014) 27, 1238–1245 1238 & 2014 USCAP, Inc All rights reserved 0893-3952/14 $32.00 Expression of cancer-testis antigens MAGEA1, MAGEA3, ACRBP, PRAME, SSX2, and CTAG2 in myxoid and round cell liposarcoma Jessica A Hemminger1, Amanda Ewart Toland2, Thomas J Scharschmidt3, Joel L Mayerson3, Denis C Guttridge2 and O Hans Iwenofu1 1Department of Pathology and Laboratory Medicine, Wexner Medical Center at The Ohio State University, Columbus, OH, USA; 2Department of Molecular Virology, Immunology and Medical Genetics, The Ohio State University Wexner Medical Center, Columbus, OH, USA and 3Department of Orthopedics, The Ohio State University Wexner Medical Center, Columbus, OH, USA Myxoid and round-cell liposarcoma is a frequently encountered liposarcoma subtype. The mainstay of treatment remains surgical excision with or without chemoradiation. However, treatment options are limited in the setting of metastatic disease. Cancer-testis antigens are immunogenic antigens with the expression largely restricted to testicular germ cells and various malignancies, making them attractive targets for cancer immunotherapy. Gene expression studies have reported the expression of various cancer-testis antigens in liposarcoma, with mRNA expression of CTAG1B, CTAG2, MAGEA9, and PRAME described specifically in myxoid and round-cell liposarcoma. Herein, we further explore the expression of the cancer-testis antigens MAGEA1, ACRBP, PRAME, and SSX2 in myxoid and round-cell liposarcoma by immunohistochemistry in addition to determining mRNA levels of CTAG2 (LAGE-1), PRAME, and MAGEA3 by quantitative real-time PCR. Samples in formalin-fixed paraffin-embedded blocks (n ¼ 37) and frozen tissue (n ¼ 8) were obtained for immunohistochemistry and quantitative real-time PCR, respectively. Full sections were stained with antibodies to MAGEA1, ACRBP, PRAME, and SSX2 and staining was assessed for intensity (1–2 þ ) and percent tumor positivity. -
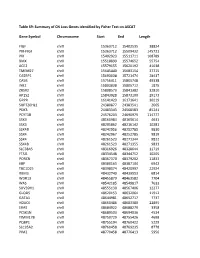
Table S9: Summary of CN Loss Genes Identified by Fisher Test on ASCAT
Table S9: Summary of CN Loss Genes identified by Fisher Test on ASCAT Gene Symbol Chromosome Start End Length FIGF chrX 15363712 15402535 38824 PIR-FIGF chrX 15363712 15509432 145721 PIR chrX 15402923 15511711 108789 BMX chrX 15518899 15574652 55754 ACE2 chrX 15579155 15620192 41038 TMEM27 chrX 15645440 15683154 37715 CA5BP1 chrX 15693038 15721474 28437 CA5B chrX 15756411 15805748 49338 INE2 chrX 15803838 15805712 1875 ZRSR2 chrX 15808573 15841382 32810 AP1S2 chrX 15843928 15873100 29173 GRPR chrX 16141423 16171641 30219 SUPT20HL1 chrX 24380877 24383541 2665 PDK3 chrX 24483343 24568583 85241 PCYT1B chrX 24576203 24690979 114777 SSX9 chrX 48160984 48165614 4631 SSX3 chrX 48205862 48216142 10281 SSX4B chrX 48242956 48252785 9830 SSX4 chrX 48242967 48252785 9819 SSX4 chrX 48261523 48271344 9822 SSX4B chrX 48261523 48271355 9833 SLC38A5 chrX 48316926 48328644 11719 FTSJ1 chrX 48334548 48344752 10205 PORCN chrX 48367370 48379202 11833 EBP chrX 48380163 48387104 6942 TBC1D25 chrX 48398074 48420997 22924 RBM3 chrX 48432740 48439553 6814 WDR13 chrX 48455879 48463582 7704 WAS chrX 48542185 48549817 7633 SUV39H1 chrX 48555130 48567406 12277 GLOD5 chrX 48620153 48632064 11912 GATA1 chrX 48644981 48652717 7737 HDAC6 chrX 48660486 48683380 22895 ERAS chrX 48684922 48688279 3358 PCSK1N chrX 48689503 48694036 4534 TIMM17B chrX 48750729 48755426 4698 PQBP1 chrX 48755194 48760422 5229 SLC35A2 chrX 48760458 48769235 8778 PIM2 chrX 48770458 48776413 5956 OTUD5 chrX 48779302 48815648 36347 KCND1 chrX 48818638 48828251 9614 GRIPAP1 chrX 48830133 48858675 28543 -

NY-ESO-1 (CTAG1B) Expression in Mesenchymal Tumors
Modern Pathology (2015) 28, 587–595 & 2015 USCAP, Inc. All rights reserved 0893-3952/15 $32.00 587 NY-ESO-1 (CTAG1B) expression in mesenchymal tumors Makoto Endo1,2,7, Marieke A de Graaff3,7, Davis R Ingram4, Simin Lim1, Dina C Lev4, Inge H Briaire-de Bruijn3, Neeta Somaiah5, Judith VMG Bove´e3, Alexander J Lazar6 and Torsten O Nielsen1 1Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, British Columbia, Canada; 2Department of Orthopaedic Surgery, Kyushu University, Fukuoka, Japan; 3Department of Pathology, Leiden University Medical Center, Leiden, The Netherlands; 4Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA; 5Department of Sarcoma Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA and 6Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA New York esophageal squamous cell carcinoma 1 (NY-ESO-1, CTAG1B) is a cancer-testis antigen and currently a focus of several targeted immunotherapeutic strategies. We performed a large-scale immunohistochemical expression study of NY-ESO-1 using tissue microarrays of mesenchymal tumors from three institutions in an international collaboration. A total of 1132 intermediate and malignant and 175 benign mesenchymal lesions were enrolled in this study. Immunohistochemical staining was performed on tissue microarrays using a monoclonal antibody for NY-ESO-1. Among mesenchymal tumors, myxoid liposarcomas showed the highest positivity for NY-ESO-1 (88%), followed by synovial sarcomas (49%), myxofibrosarcomas (35%), and conventional chondrosarcomas (28%). Positivity of NY-ESO-1 in the remaining mesenchymal tumors was consistently low, and no immunoreactivity was observed in benign mesenchymal lesions. -

Small Cell Carcinoma of the Ovary, Hypercalcemic Type (SCCOHT) Beyond SMARCA4 Mutations: a Comprehensive Genomic Analysis
cells Article Small Cell Carcinoma of the Ovary, Hypercalcemic Type (SCCOHT) beyond SMARCA4 Mutations: A Comprehensive Genomic Analysis Aurélie Auguste 1,Félix Blanc-Durand 2 , Marc Deloger 3 , Audrey Le Formal 1, Rohan Bareja 4,5, David C. Wilkes 4 , Catherine Richon 6,Béatrice Brunn 2, Olivier Caron 6, Mojgan Devouassoux-Shisheboran 7,Sébastien Gouy 2, Philippe Morice 2, Enrica Bentivegna 2, Andrea Sboner 4,5,8, Olivier Elemento 4,8, Mark A. Rubin 9 , Patricia Pautier 2, Catherine Genestie 10, Joanna Cyrta 4,9,11 and Alexandra Leary 1,2,* 1 Medical Oncologist, Gynecology Unit, Lead Translational Research Team, INSERM U981, Gustave Roussy, 94805 Villejuif, France; [email protected] (A.A.); [email protected] (A.L.F.) 2 Gynecological Unit, Department of Medicine, Gustave Roussy, 94805 Villejuif, France; [email protected] (F.B.-D.); [email protected] (B.B.); [email protected] (S.G.); [email protected] (P.M.); [email protected] (E.B.); [email protected] (P.P.) 3 Bioinformatics Core Facility, Gustave Roussy Cancer Center, UMS CNRS 3655/INSERM 23 AMMICA, 94805 Villejuif, France; [email protected] 4 Caryl and Israel Englander Institute for Precision Medicine, Weill Cornell Medicine, New York, NY 10001, USA; [email protected] (R.B.); [email protected] (D.C.W.); [email protected] (A.S.); [email protected] (O.E.); [email protected] (J.C.) 5 Institute for Computational Biomedicine, Weill Cornell -

Identification of Differentially Methylated Cpg
biomedicines Article Identification of Differentially Methylated CpG Sites in Fibroblasts from Keloid Scars Mansour A. Alghamdi 1,2 , Hilary J. Wallace 3,4 , Phillip E. Melton 5,6 , Eric K. Moses 5,6, Andrew Stevenson 4 , Laith N. Al-Eitan 7,8 , Suzanne Rea 9, Janine M. Duke 4, 10 11 4,9,12 4, , Patricia L. Danielsen , Cecilia M. Prêle , Fiona M. Wood and Mark W. Fear * y 1 Department of Anatomy, College of Medicine, King Khalid University, Abha 61421, Saudi Arabia; [email protected] 2 Genomics and Personalized Medicine Unit, College of Medicine, King Khalid University, Abha 61421, Saudi Arabia 3 School of Medicine, The University of Notre Dame Australia, Fremantle 6959, Australia; [email protected] 4 Burn Injury Research Unit, School of Biomedical Sciences, Faculty of Health and Medical Sciences, The University of Western Australia, Perth 6009, Australia; andrew@fionawoodfoundation.com (A.S.); [email protected] (J.M.D.); fi[email protected] (F.M.W.) 5 Centre for Genetic Origins of Health and Disease, Faculty of Health and Medical Sciences, The University of Western Australia, Perth 6009, Australia; [email protected] (P.E.M.); [email protected] (E.K.M.) 6 School of Pharmacy and Biomedical Sciences, Faculty of Health Science, Curtin University, Perth 6102, Australia 7 Department of Applied Biological Sciences, Jordan University of Science and Technology, Irbid 22110, Jordan; [email protected] 8 Department of Biotechnology and Genetic Engineering, Jordan University of Science and Technology, Irbid 22110, -

Gene Expression Profiling Using Nanostring Digital RNA Counting to Identify Potential Target Antigens for Melanoma Immunotherapy
Published OnlineFirst September 10, 2013; DOI: 10.1158/1078-0432.CCR-13-1253 Clinical Cancer Human Cancer Biology Research Gene Expression Profiling using Nanostring Digital RNA Counting to Identify Potential Target Antigens for Melanoma Immunotherapy Rachel E. Beard, Daniel Abate-Daga, Shannon F. Rosati, Zhili Zheng, John R. Wunderlich, Steven A. Rosenberg, and Richard A. Morgan Abstract Purpose: The success of immunotherapy for the treatment of metastatic cancer is contingent on the identification of appropriate target antigens. Potential targets must be expressed on tumors but show restricted expression on normal tissues. To maximize patient eligibility, ideal target antigens should be expressed on a high percentage of tumors within a histology and, potentially, in multiple different malignancies. Design: A Nanostring probeset was designed containing 97 genes, 72 of which are considered potential candidate genes for immunotherapy. Five established melanoma cell lines, 59 resected metastatic mela- noma tumors, and 31 normal tissue samples were profiled and analyzed using Nanostring technology. Results: Of the 72 potential target genes, 33 were overexpressed in more than 20% of studied melanoma tumor samples. Twenty of those genes were identified as differentially expressed between normal tissues and tumor samples by ANOVA analysis. Analysis of normal tissue gene expression identified seven genes with limited normal tissue expression that warrant further consideration as potential immunotherapy target antigens: CSAG2, MAGEA3, MAGEC2, IL13RA2, PRAME, CSPG4, and SOX10. These genes were highly overexpressed on a large percentage of the studied tumor samples, with expression in a limited number of normal tissue samples at much lower levels. Conclusion: The application of Nanostring RNA counting technology was used to directly quantitate the gene expression levels of multiple potential tumor antigens.