Laboratory Approach to the Diagnosis of Smallpox: Module 2 – Poxvirus Overview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Poxviruses: Smallpox Vaccine, Its Complications and Chemotherapy
Virus Adaptation and Treatment Dovepress open access to scientific and medical research Open Access Full Text Article R E V IEW Poxviruses: smallpox vaccine, its complications and chemotherapy Mimi Remichkova Abstract: The threat of bioterrorism in the recent years has once again posed to mankind the unresolved problems of contagious diseases, well forgotten in the past. Smallpox (variola) is Department of Pathogenic Bacteria, The Stephan Angeloff Institute among the most dangerous and highly contagious viral infections affecting humans. The last of Microbiology, Bulgarian Academy natural case in Somalia marked the end of a successful World Health Organization campaign of Sciences, Sofia, Bulgaria for smallpox eradication by vaccination on worldwide scale. Smallpox virus still exists today in some laboratories, specially designated for that purpose. The contemporary response in the treatment of the post-vaccine complications, which would occur upon enforcing new programs for mass-scale smallpox immunization, includes application of effective chemotherapeutics and their combinations. The goals are to provide the highest possible level of protection and safety of For personal use only. the population in case of eventual terrorist attack. This review describes the characteristic features of the poxviruses, smallpox vaccination, its adverse reactions, and poxvirus chemotherapy. Keywords: poxvirus, smallpox vaccine, post vaccine complications, inhibitors Characteristics of poxviruses Smallpox (variola) infection is caused by the smallpox virus. This virus belongs to the genus of Orthopoxvirus included in the Poxviridae family. Poxviruses are one of the largest and most complexly structured viruses, known so far. The genome of poxviruses consists of a linear two-chained DNA and its replication takes place in the cytoplasm of the infected cell. -

Vaccinia Belongs to a Family of Viruses That Is Closely Related to the Smallpox Virus
VACCINIA INFECTION What is it? Vaccinia belongs to a family of viruses that is closely related to the smallpox virus. Because of the similarities between the smallpox and vaccinia viruses, the vaccinia virus is used in the smallpox vaccine. When this virus is used as a vaccine, it allows our immune systems to develop immunity against smallpox. The smallpox vaccine does not actually contain smallpox virus and cannot cause smallpox. Vaccination usually prevents smallpox infection for at least ten years. The vaccinia vaccine against smallpox was used to successfully eradicate smallpox from the human population. More recently, this virus has also become of interest due to concerns about smallpox being used as an agent of bioterrorism. How is the virus spread? Vaccinia can be spread by touching the vaccination site before it has fully healed or by touching clothing or bandages that have been contaminated with the live virus during vaccination. In this manner, vaccinia can spread to other parts of the body and to other individuals. It cannot be spread through the air. What are the symptoms of vaccinia? Vaccinia virus symptoms are similar to smallpox, but milder. Vaccinia may cause rash, fever, headache and body aches. In certain individuals, such as those with weak immune systems, the symptoms can be more severe. What are the potential side effects of the vaccinia vaccine for smallpox? Normal reactions are mild and go away without any treatment.These include: Soreness and redness in the arm where the vaccine was given Slightly swollen, sore glands in the armpits Low grade fever One in approximately three people will feel badly enough to miss school, work or recreational activities Trouble sleeping Serious reactions are not very common but can occur in about 1,000 in every 1 million people who are vaccinated for the first time. -
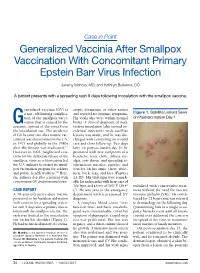
Generalized Vaccinia After Smallpox Vaccination with Concomitant Primary Epstein Barr Virus Infection
Case in Point Generalized Vaccinia After Smallpox Vaccination With Concomitant Primary Epstein Barr Virus Infection Jeremy Mandia, MD; and Kathryn Buikema, DO A patient presents with a spreading rash 9 days following inoculation with the smallpox vaccine. eneralized vaccinia (GV) is atopic dermatitis, or other rashes a rare, self-limiting complica- and reported no systemic symptoms. Figure 1. Satellite Lesions Seen tion of the smallpox vacci- His vitals also were within normal on Postvaccination Day 1 Gnation that is caused by the limits. A clinical diagnosis of inad- systemic spread of the virus from vertent inoculation (also termed ac- the inoculation site. The incidence cidental infection) with satellite of GV became rare after routine vac- lesions was made, and he was dis- cination was discontinued in the U.S. charged with counseling on wound in 1971 and globally in the 1980s care and close follow-up. Two days after the disease was eradicated.1,2 later, on postvaccination day 11, he However in 2002, heightened con- presented with new symptoms of a cerns for the deliberate release of the headache, fever, chills, diffuse my- smallpox virus as a bioweapon led algia, sore throat, and spreading er- the U.S. military to restart its small- ythematous macules, papules, and pox vaccination program for soldiers vesicles on his arms, chest, abdo- and public health workers.3,4 Here, men, back, legs, and face (Figures the authors describe a patient with 2A-2D). His vital signs were remark- concomitant GV and mononucleosis. able for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º stabilized with conservative treat- CASE REPORT C). -

Cidofovir Activity Against Poxvirus Infections
Viruses 2010 , 2, 2803-2830; doi:10.3390/v2122803 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review Cidofovir Activity against Poxvirus Infections Graciela Andrei * and Robert Snoeck Laboratory of Virology and Chemotherapy, Rega Institute for Medical Research, KULeuven, Minderboredersstraat 10, B-3000 Leuven, Belgium; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +32-16-337372; Fax: +32-16-337340. Received: 10 November 2010; in revised form: 9 December 2010 / Accepted: 10 December 2010 / Published: 22 December 2010 Abstract: Cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, HPMPC] is an acyclic nucleoside analog approved since 1996 for clinical use in the treatment of cytomegalovirus (CMV) retinitis in AIDS patients. Cidofovir (CDV) has broad-spectrum activity against DNA viruses, including herpes-, adeno-, polyoma-, papilloma- and poxviruses. Among poxviruses, cidofovir has shown in vitro activity against orthopox [vaccinia, variola (smallpox), cowpox, monkeypox, camelpox, ectromelia], molluscipox [molluscum contagiosum] and parapox [orf] viruses. The anti-poxvirus activity of cidofovir in vivo has been shown in different models of infection when the compound was administered either intraperitoneal, intranasal (aerosolized) or topically. In humans, cidofovir has been successfully used for the treatment of recalcitrant molluscum contagiosum virus and orf virus in immunocompromised patients. CDV remains a reference compound against poxviruses and holds potential for the therapy and short-term prophylaxis of not only orthopox- but also parapox- and molluscipoxvirus infections. Keywords: cidofovir; poxviruses; acyclic nucleoside analog 1. Introduction The antiviral activity of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC, cidofovir, CDV) (Figure 1) against human cytomegalovirus (HCMV) and other DNA viruses was first Viruses 2010 , 2 2804 reported in 1986 [1]. -

ICD-9 Diagnosis Codes Effective 10/1/2011 (V29.0) Source: Centers for Medicare and Medicaid Services
ICD-9 Diagnosis Codes effective 10/1/2011 (v29.0) Source: Centers for Medicare and Medicaid Services 0010 Cholera d/t vib cholerae 00801 Int inf e coli entrpath 01086 Prim prg TB NEC-oth test 0011 Cholera d/t vib el tor 00802 Int inf e coli entrtoxgn 01090 Primary TB NOS-unspec 0019 Cholera NOS 00803 Int inf e coli entrnvsv 01091 Primary TB NOS-no exam 0020 Typhoid fever 00804 Int inf e coli entrhmrg 01092 Primary TB NOS-exam unkn 0021 Paratyphoid fever a 00809 Int inf e coli spcf NEC 01093 Primary TB NOS-micro dx 0022 Paratyphoid fever b 0081 Arizona enteritis 01094 Primary TB NOS-cult dx 0023 Paratyphoid fever c 0082 Aerobacter enteritis 01095 Primary TB NOS-histo dx 0029 Paratyphoid fever NOS 0083 Proteus enteritis 01096 Primary TB NOS-oth test 0030 Salmonella enteritis 00841 Staphylococc enteritis 01100 TB lung infiltr-unspec 0031 Salmonella septicemia 00842 Pseudomonas enteritis 01101 TB lung infiltr-no exam 00320 Local salmonella inf NOS 00843 Int infec campylobacter 01102 TB lung infiltr-exm unkn 00321 Salmonella meningitis 00844 Int inf yrsnia entrcltca 01103 TB lung infiltr-micro dx 00322 Salmonella pneumonia 00845 Int inf clstrdium dfcile 01104 TB lung infiltr-cult dx 00323 Salmonella arthritis 00846 Intes infec oth anerobes 01105 TB lung infiltr-histo dx 00324 Salmonella osteomyelitis 00847 Int inf oth grm neg bctr 01106 TB lung infiltr-oth test 00329 Local salmonella inf NEC 00849 Bacterial enteritis NEC 01110 TB lung nodular-unspec 0038 Salmonella infection NEC 0085 Bacterial enteritis NOS 01111 TB lung nodular-no exam 0039 -

List of Codes Used to Identify Measures Reported in the QDFC
List of Codes Used to Identify Measures Reported in the Quarterly Dialysis Facility Compare Reports July 2018 List of Codes Used to Identify Measures Reported in the Quarterly Dialysis Facility Compare Reports Table of Contents Table 1a: Transfusion Summary for Medicare Dialysis Patients, Codes Used for Exclusions 3 CARCINOMA 3 COAGULATION 5 HEAD/NECK CANCER 5 HEMOLYTIC OR APLASTIC ANEMIA 9 LEUKEMIA 11 LYMPHOMA 15 METASTATIC 27 MYELOMA, ETC. 29 OTHER CANCER 30 SICKLE CELL 34 SOLID ORGAN CANCER 34 Table 1b: Transfusion Summary for Medicare Dialysis Patients, Codes Used to Identify Transfusion Events .................................................................................................................. 45 REVENUE CENTER CODES 45 PROCEDURE CODES 45 VALUE CODES 46 HCPCS CODE 46 Table 2a: Vascular Access Measures (SFR and Long-Term Catheter) for Medicare Dialysis Patients Based on Medicare Claims, Codes Used for Exclusions ........................................... 46 Produced by The University of Michigan Kidney Epidemiology and Cost Center Page 1 of 135 List of Codes Used to Identify Measures Reported in the Quarterly Dialysis Facility Compare Reports July 2018 COMA 46 END STAGE LIVER DISEASE 48 METASTATIC CANCER 48 Table 2b: Standardized Fistulae Rate (SFR) for Medicare Dialysis Patients Based on Medicare Claims, Codes Used for Prevalent Comorbidities Adjusted in Model .................................... 50 ANEMIA 50 CORONARY ARTERY DISEASE 52 CONGESTIVE HEART FAILURE 55 CEREBROVASCULAR DISEASE 56 CHRONIC OBSTRUCTIVE PULMONARY DISEASE 68 DIABETES 69 DRUG DEPENDENCE 79 INFECTIONS (NON-VASCULAR ACCESS-RELATED): 93 PERIPHERAL VASCULAR DISEASE (INCLUDES ARTERIAL, VENOUS AND NONSPECIFIC DISEASES) 124 Table 3: Dialysis Adequacy ................................................................................................... -

Medical Management of Biological Casualties Handbook
USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Sixth Edition April 2005 U.S. ARMY MEDICAL RESEARCH INSTITUTE OF INFECTIOUS DISEASES FORT DETRICK FREDERICK, MARYLAND Emergency Response Numbers National Response Center: 1-800-424-8802 or (for chem/bio hazards & terrorist events) 1-202-267-2675 National Domestic Preparedness Office: 1-202-324-9025 (for civilian use) Domestic Preparedness Chem/Bio Helpline: 1-410-436-4484 or (Edgewood Ops Center – for military use) DSN 584-4484 USAMRIID’s Emergency Response Line: 1-888-872-7443 CDC'S Emergency Response Line: 1-770-488-7100 Handbook Download Site An Adobe Acrobat Reader (pdf file) version of this handbook can be downloaded from the internet at the following url: http://www.usamriid.army.mil USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Sixth Edition April 2005 Lead Editor Lt Col Jon B. Woods, MC, USAF Contributing Editors CAPT Robert G. Darling, MC, USN LTC Zygmunt F. Dembek, MS, USAR Lt Col Bridget K. Carr, MSC, USAF COL Ted J. Cieslak, MC, USA LCDR James V. Lawler, MC, USN MAJ Anthony C. Littrell, MC, USA LTC Mark G. Kortepeter, MC, USA LTC Nelson W. Rebert, MS, USA LTC Scott A. Stanek, MC, USA COL James W. Martin, MC, USA Comments and suggestions are appreciated and should be addressed to: Operational Medicine Department Attn: MCMR-UIM-O U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) Fort Detrick, Maryland 21702-5011 PREFACE TO THE SIXTH EDITION The Medical Management of Biological Casualties Handbook, which has become affectionately known as the "Blue Book," has been enormously successful - far beyond our expectations. -

Client Services Manual Public Health Laboratory
CLIENT SERVICES MANUAL PUBLIC HEALTH LABORATORY COUNTY OF SANTA CLARA 2220 MOORPARK AVE, 2ND FLOOR SAN JOSE, CA 95128 (P) 408.885.4272 | (F) 408.885.4275 http://www.sccgov.org/sites /sccphd/en-us/HealthProviders/Lab Patricia Dadone, Public Health Laboratory Director Sara H. Cody, MD, Health Officer and Public Health Director Table of Contents 1 GENERAL INFORMATION ............................................................................................... 1.1 ROLE .............................................................................................................................................................. 1.1 MISSION STATEMENT ..................................................................................................................................... 1.1 ABBREVIATIONS.............................................................................................................................................. 1.2 LABORATORY CERTIFICATIONS ........................................................................................................................ 1.4 CLIENT SERVICES ............................................................................................................................................ 1.5 Hours of Operation: .............................................................................................................................. 1.5 Supplies .................................................................................................................................................. -

Vaccinia Virus
APPENDIX 2 Vaccinia Virus • Accidental infection following transfer from the vac- cination site to another site (autoinoculation) or to Disease Agent: another person following intimate contact Likelihood of Secondary Transmission: • Vaccinia virus • Significant following direct contact Disease Agent Characteristics: At-Risk Populations: • Family: Poxviridae; Subfamily: Chordopoxvirinae; • Individuals receiving smallpox (vaccinia) vaccination Genus: Orthopoxvirus • Individuals who come in direct contact with vacci- • Virion morphology and size: Enveloped, biconcave nated persons core with two lateral bodies, brick-shaped to pleo- • Those at risk for more severe complications of infec- morphic virions, ~360 ¥ 270 ¥ 250 nm in size tion include the following: • Nucleic acid: Nonsegmented, linear, covalently ᭺ Immune-compromised persons including preg- closed, double-stranded DNA, 18.9-20.0 kb in length nant women • Physicochemical properties: Virus is inactivated at ᭺ Patients with atopy, especially those with eczema 60°C for 8 minutes, but antigen can withstand 100°C; ᭺ Patients with extensive exfoliative skin disease lyophilized virus maintains potency for 18 months at 4-6°C; virus may be stable when dried onto inanimate Vector and Reservoir Involved: surfaces; susceptible to 1% sodium hypochlorite, • No natural host 2% glutaraldehyde, and formaldehyde; disinfection of hands and environmental contamination with soap Blood Phase: and water are effective • Vaccinia DNA was detected by PCR in the blood in 6.5% of 77 military members from 1 to 3 weeks after Disease Name: smallpox (vaccinia) vaccination that resulted in a major skin reaction. • Progressive vaccinia (vaccinia necrosum or vaccinia • In the absence of complications after immunization, gangrenosum) recently published PCR and culture data suggest that • Generalized vaccinia viremia with current vaccines must be rare 3 weeks • Eczema vaccinatum after vaccination. -

Specimen Type, Collection Methods, and Diagnostic Assays Available For
Specimen type, collection methods, and diagnostic assays available for the detection of poxviruses from human specimens by the Poxvirus and Rabies Branch, Centers for Disease Control and Prevention1. Specimen Orthopoxvirus Parapoxvirus Yatapoxvirus Molluscipoxvirus Specimen type collection method PCR6 Culture EM8 IHC9,10 Serology11 PCR12 EM8 IHC9,10 PCR13 EM8 PCR EM8 Lesion material Fresh or frozen Swab 5 Lesion material [dry or in media ] [vesicle / pustule Formalin fixed skin, scab / crust, etc.] Paraffin block Fixed slide(s) Container Lesion fluid Swab [vesicle / pustule [dry or in media5] fluid, etc.] Touch prep slide Blood EDTA2 EDTA tube 7 Spun or aliquoted Serum before shipment Spun or aliquoted Plasma before shipment CSF3,4 Sterile 1. The detection of poxviruses by electron microscopy (EM) and immunohistochemical staining (IHC) is performed by the Infectious Disease Pathology Branch of the CDC. 2. EDTA — Ethylenediaminetetraacetic acid. 3. CSF — Cerebrospinal fluid. 4. In order to accurately interpret test results generated from CSF specimens, paired serum must also be submitted. 5. If media is used to store and transport specimens a minimal amount should be used to ensure as little dilution of DNA as possible. 6. Orthopoxvirus generic real-time polymerase chain reaction (PCR) assays will amplify DNA from numerous species of virus within the Orthopoxvirus genus. Species-specific real-time PCR assays are available for selective detection of DNA from variola virus, vaccinia virus, monkeypox virus, and cowpox virus. 7. Blood is not ideal for the detection of orthopoxviruses by PCR as the period of viremia has often passed before sampling occurs. 8. EM can reveal the presence of a poxvirus in clinical specimens or from virus culture, but this technique cannot differentiate between virus species within the same genus. -

(12) Patent Application Publication (10) Pub. No.: US 2012/0009150 A1 WEBER Et Al
US 2012O009 150A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0009150 A1 WEBER et al. (43) Pub. Date: Jan. 12, 2012 (54) DIARYLUREAS FORTREATINGVIRUS Publication Classification INFECTIONS (51) Int. Cl. (76) Inventors: Olaf WEBER, Wulfrath (DE); st 2. CR Bernd Riedl, Wuppertal (DE) ( .01) A63/675 (2006.01) (21) Appl. No.: 13/236,865 A6II 3/522 (2006.01) A6IP 29/00 (2006.01) (22) Filed: Sep. 20, 2011 A6II 3/662 (2006.01) A638/14 (2006.01) Related U.S. Application Data A63L/7056 (2006.01) A6IP3L/2 (2006.01) (63) Continuation of application No. 12/097.350. filed on A6II 3/44 (2006.01) Nov. 3, 2008, filed as application No. PCTAEPO6/ A6II 3/52 (2006.01) 11693 on Dec. 6, 2006. O O (52) U.S. Cl. .......... 424/85.6; 514/350; 514/171; 514/81; (30) Foreign Application Priority Data 514/263.38: 514/263.4: 514/120: 514/4.3: Dec. 15, 2005 (EP) .................................. 05O274513 424/85.7; 514/43 Dec. 15, 2005 (EP). ... O5O27452.1 Dec. 15, 2005 (EP). ... O5O27456.2 Dec. 15, 2005 (EP). ... O5O27458.8 The present invention relates to pharmaceutical compositions Dec. 15, 2005 (EP) O5O27.460.4 for treating virus infections and/or diseases caused by virus Dec. 15, 2005 (EP) O5O27462.O infections comprising at least a diary1 urea compound option Dec. 15, 2005 (EP). ... O5O27465.3 ally combined with at least one additional therapeutic agent. Dec. 15, 2005 (EP). ... O5O274.67.9 Useful combinations include e.g. BAY 43-9006 as a diaryl Dec. -

Harpercollins Books for the First-Year Student
S t u d e n t Featured Titles • American History and Society • Food, Health, and the Environment • World Issues • Memoir/World Views • Memoir/ American Voices • World Fiction • Fiction • Classic Fiction • Religion • Orientation Resources • Inspiration/Self-Help • Study Resources www.HarperAcademic.com Index View Print Exit Books for t H e f i r s t - Y e A r s t u d e n t • • 1 FEATURED TITLES The Boy Who Harnessed A Pearl In the Storm the Wind How i found My Heart in tHe Middle of tHe Ocean Creating Currents of eleCtriCity and Hope tori Murden McClure William kamkwamba & Bryan Mealer During June 1998, Tori Murden McClure set out to William Kamkwamba was born in Malawi, Africa, a row across the Atlantic Ocean by herself in a twenty- country plagued by AIDS and poverty. When, in three-foot plywood boat with no motor or sail. 2002, Malawi experienced their worst famine in 50 Within days she lost all communication with shore, years, fourteen-year-old William was forced to drop ultimately losing updates on the location of the Gulf out of school because his family could not afford the Stream and on the weather. In deep solitude and $80-a-year-tuition. However, he continued to think, perilous conditions, she was nonetheless learn, and dream. Armed with curiosity, determined to prove what one person with a mission determination, and a few old science textbooks he could do. When she was finally brought to her knees discovered in a nearby library, he embarked on a by a series of violent storms that nearly killed her, daring plan to build a windmill that could bring his she had to signal for help and go home in what felt family the electricity only two percent of Malawians like complete disgrace.