Plant Cytokinesis: Terminology for Structures and Processes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phragmoplast Microtubule Dynamics – a Game of Zones Andrei Smertenko1,2,‡, Seanna L
© 2018. Published by The Company of Biologists Ltd | Journal of Cell Science (2018) 131, jcs203331. doi:10.1242/jcs.203331 REVIEW SPECIAL ISSUE: PLANT CELL BIOLOGY Phragmoplast microtubule dynamics – a game of zones Andrei Smertenko1,2,‡, Seanna L. Hewitt2,3, Caitlin N. Jacques2,4, Rafal Kacprzyk1, Yan Liu2,5, Matthew J. Marcec2,6, Lindani Moyo2,6, Aaron Ogden1,2, Hui Min Oung1,2, Sharol Schmidt1,2 and Erika A. Serrano-Romero2,5 ABSTRACT during anaphase from the remnants of the central spindle (Segui- Plant morphogenesis relies on the accurate positioning of the partition Simarro et al., 2004). It consists of microtubules, actin, membrane (cell plate) between dividing cells during cytokinesis. The cell plate is compartments and proteins that associate with or regulate the above synthetized by a specialized structure called the phragmoplast, which (Boruc and Van Damme, 2015; Lipka et al., 2015). The microtubule consists of microtubules, actin filaments, membrane compartments component of the phragmoplast consists of two aligned arrays that and associated proteins. The phragmoplast forms between daughter flank the so-called phragmoplast midzone, where cell plate assembly nuclei during the transition from anaphase to telophase. As cells are takes place (Fig. 1). The initial phragmoplast has a disk shape with a commonly larger than the originally formed phragmoplast, the diameter that approximately equals that of the daughter nuclei construction of the cell plate requires phragmoplast expansion. (Fig. 1); however, the parental cell is generally wider. For example, This expansion depends on microtubule polymerization at the the length of a cambium cell exceeds the diameter of the disk-shaped phragmoplast forefront (leading zone) and loss at the back (lagging phragmoplast during late anaphase by up to 100 fold (Larson, 1994). -

The Revised Classification of Eukaryotes
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/231610049 The Revised Classification of Eukaryotes Article in Journal of Eukaryotic Microbiology · September 2012 DOI: 10.1111/j.1550-7408.2012.00644.x · Source: PubMed CITATIONS READS 961 2,825 25 authors, including: Sina M Adl Alastair Simpson University of Saskatchewan Dalhousie University 118 PUBLICATIONS 8,522 CITATIONS 264 PUBLICATIONS 10,739 CITATIONS SEE PROFILE SEE PROFILE Christopher E Lane David Bass University of Rhode Island Natural History Museum, London 82 PUBLICATIONS 6,233 CITATIONS 464 PUBLICATIONS 7,765 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Biodiversity and ecology of soil taste amoeba View project Predator control of diversity View project All content following this page was uploaded by Smirnov Alexey on 25 October 2017. The user has requested enhancement of the downloaded file. The Journal of Published by the International Society of Eukaryotic Microbiology Protistologists J. Eukaryot. Microbiol., 59(5), 2012 pp. 429–493 © 2012 The Author(s) Journal of Eukaryotic Microbiology © 2012 International Society of Protistologists DOI: 10.1111/j.1550-7408.2012.00644.x The Revised Classification of Eukaryotes SINA M. ADL,a,b ALASTAIR G. B. SIMPSON,b CHRISTOPHER E. LANE,c JULIUS LUKESˇ,d DAVID BASS,e SAMUEL S. BOWSER,f MATTHEW W. BROWN,g FABIEN BURKI,h MICAH DUNTHORN,i VLADIMIR HAMPL,j AARON HEISS,b MONA HOPPENRATH,k ENRIQUE LARA,l LINE LE GALL,m DENIS H. LYNN,n,1 HILARY MCMANUS,o EDWARD A. D. -

Molecular Biology and Applied Genetics
MOLECULAR BIOLOGY AND APPLIED GENETICS FOR Medical Laboratory Technology Students Upgraded Lecture Note Series Mohammed Awole Adem Jimma University MOLECULAR BIOLOGY AND APPLIED GENETICS For Medical Laboratory Technician Students Lecture Note Series Mohammed Awole Adem Upgraded - 2006 In collaboration with The Carter Center (EPHTI) and The Federal Democratic Republic of Ethiopia Ministry of Education and Ministry of Health Jimma University PREFACE The problem faced today in the learning and teaching of Applied Genetics and Molecular Biology for laboratory technologists in universities, colleges andhealth institutions primarily from the unavailability of textbooks that focus on the needs of Ethiopian students. This lecture note has been prepared with the primary aim of alleviating the problems encountered in the teaching of Medical Applied Genetics and Molecular Biology course and in minimizing discrepancies prevailing among the different teaching and training health institutions. It can also be used in teaching any introductory course on medical Applied Genetics and Molecular Biology and as a reference material. This lecture note is specifically designed for medical laboratory technologists, and includes only those areas of molecular cell biology and Applied Genetics relevant to degree-level understanding of modern laboratory technology. Since genetics is prerequisite course to molecular biology, the lecture note starts with Genetics i followed by Molecular Biology. It provides students with molecular background to enable them to understand and critically analyze recent advances in laboratory sciences. Finally, it contains a glossary, which summarizes important terminologies used in the text. Each chapter begins by specific learning objectives and at the end of each chapter review questions are also included. -

Functions of the Arabidopsis Kinesin Superfamily of Microtubule-Based Motor Proteins Chuanmei Zhu
Washington University in St. Louis Washington University Open Scholarship Biology Faculty Publications & Presentations Biology 10-2012 Functions of the Arabidopsis kinesin superfamily of microtubule-based motor proteins Chuanmei Zhu Ram Dixit Washington University in St Louis, [email protected] Follow this and additional works at: https://openscholarship.wustl.edu/bio_facpubs Part of the Biochemistry Commons, Biology Commons, and the Plant Biology Commons Recommended Citation Zhu, Chuanmei and Dixit, Ram, "Functions of the Arabidopsis kinesin superfamily of microtubule-based motor proteins" (2012). Biology Faculty Publications & Presentations. 79. https://openscholarship.wustl.edu/bio_facpubs/79 This Article is brought to you for free and open access by the Biology at Washington University Open Scholarship. It has been accepted for inclusion in Biology Faculty Publications & Presentations by an authorized administrator of Washington University Open Scholarship. For more information, please contact [email protected]. Functions of the Arabidopsis kinesin superfamily of microtubule-based motor proteins Chuanmei Zhu and Ram Dixit Biology Department, Washington University, St. Louis, MO 63130 Corresponding author Ram Dixit 1 Brookings Drive, CB 1137 St. Louis, MO 63130. Phone: (314) 935-8823 Fax: (314) 935-4432 Email: [email protected] Keywords Plant, cortical microtubule, preprophase band, spindle, phragmoplast ABSTRACT Plants possess a large number of microtubule-based kinesin motor proteins. While the Kinesin-2, 3, 9 and 11 families are absent from land plants, the Kinesin-7 and 14 families are greatly expanded. In addition, some kinesins are specifically present only in land plants. The distinctive inventory of plant kinesins suggests that kinesins have evolved to perform specialized functions in plants. -

Rangap1 Is a Continuous Marker of the Arabidopsis Cell Division Plane
RanGAP1 is a continuous marker of the Arabidopsis cell division plane Xianfeng Morgan Xua,1, Qiao Zhaoa,2, Thushani Rodrigo-Peirisa, Jelena Brkljacica, Chao Sylvia Hea,1, Sabine Mu¨ llerb, and Iris Meiera,3 aPlant Biotechnology Center and Department of Plant Cellular and Molecular Biology, The Ohio State University, Columbus, OH 43210; and bSchool of Biological Sciences, University of Auckland, Auckland 1142, New Zealand Edited by Maarten J. Chrispeels, University of California at San Diego, La Jolla, CA, and approved October 8, 2008 (received for review June 30, 2008) In higher plants, the plane of cell division is faithfully predicted by were found to interact with TANGLED and a pok1 pok2 double the preprophase band (PPB). The PPB, a cortical ring of microtu- mutant resembles the maize tan mutant in terms of misoriented bules and F-actin, disassembles upon nuclear-envelope break- division planes (9). Although the data suggest a role for kinesins down. During cytokinesis, the expanding cell plate fuses with the and the pioneer protein TANGLED in division-plane definition, plasma membrane at the cortical division site, the site of the former the molecular mechanism of the process remains unknown. PPB. The nature of the ‘‘molecular memory’’ that is left behind by Ran is a small GTPase that in vertebrates controls multiple the PPB and is proposed to guide the cell plate to the cortical cellular processes including nucleocytoplasmic transport, spindle division site is unknown. RanGAP is the GTPase activating protein assembly, nuclear envelope reassembly, centrosome duplication, of the small GTPase Ran, which provides spatial information for and cell-cycle control (ref. -
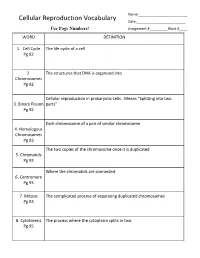
Cellular Reproduction Vocabulary Date:______Use Page Numbers! Assignment #______Block #____ WORD DEFINITION
Name:_________________________ Cellular Reproduction Vocabulary Date:_________________________ Use Page Numbers! Assignment #_________ Block #____ WORD DEFINITION 1. Cell Cycle The life cycle of a cell Pg 92 2. The structures that DNA is organized into Chromosomes Pg 92 Cellular reproduction in prokaryotic cells. Means “Splitting into two 3. Binary Fission parts” Pg 92 Each chromosome of a pair of similar chromosome 4. Homologous Chromosomes Pg 93 The two copies of the chromosome once it is duplicated 5. Chromatids Pg 93 Where the chromatids are connected 6. Centromere Pg 93 7. Mitosis The complicated process of separating duplicated chromosomes Pg 93 8. Cytokinesis The process where the cytoplasm splits in two Pg 95 WORD DEFINITION 9. Budding A type of asexual reproduction, where a piece of the parents body Pg 612 develops into an independent organism 10. A type of asexual reproduction, where the organism breaks into two or Regeneration more parts, each growing into a new organism that is genetically identical (fragmentation) to the parent Pg 612 The production of offspring by combining the genetic material of more 11. Sexual than one parent reproduction Pg 613 A single parent has offspring that are identical to itself 12. Asexual reproduction Pg 613 The male sex cell 13. Sperm Pg 613 The female sex cell 14. Egg Pg 613 The new type of cell that is made when an egg’s nucleus fuses with a 15. Zygote sperm’s nucleus Pg 613 16. Spores Small reproductive cells that are protected by a thick cell wall Pg 256 WORD DEFINITION 17. Sex Cells Specialized cells that combine to form a zygote, they have half the normal Pg 114 number of chromosomes, one of each pair. -

KCH Kinesin Drives Nuclear Transport and Cytoskeletal Coalescence for Tip Cell Growth
bioRxiv preprint doi: https://doi.org/10.1101/308775; this version posted April 26, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. KCH kinesin drives nuclear transport and cytoskeletal coalescence for tip cell growth Moé Yamada and Gohta Goshima# Division of Biological Science, Graduate School of Science, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8602, Japan #[email protected] Long-distance transport along including organelles, proteins, and RNA, microtubules (MTs) is critical for are transported to their appropriate intracellular organisation. In animals, positions where they specifically function in antagonistic motor proteins kinesin response to internal and external signals. (plus end-directed) and dynein (minus Although it had been believed that plants end-directed) drive cargo transport. In predominantly utilize actin and myosin to land plants, however, the identity of move cellular components, recent studies motors responsible for transport is have uncovered the prevalence of poorly understood, as genes encoding microtubule (MT)-dependent transport as cytoplasmic dynein are missing. How well (Kong et al., 2015; Miki et al., 2015; other functions of dynein are brought Nakaoka et al., 2015; Zhu et al., 2015; about in plants also remains unknown. Yamada et al., 2017). However, a unique Here, we show that a subclass of the feature of plant motor systems is that the kinesin-14 family, KCH—which can also genes encoding cytoplasmic dynein, the bind actin—drives MT minus sole MT minus end-directed transporter in end-directed nuclear transport in the animals, have been lost during plant moss Physcomitrella patens. -

Identification and Characterization of the Land-Plant-Specific Microtubule Nucleation Factor MACET4 Sharol Schmidt and Andrei Smertenko*
© 2019. Published by The Company of Biologists Ltd | Journal of Cell Science (2019) 132, jcs232819. doi:10.1242/jcs.232819 RESEARCH ARTICLE Identification and characterization of the land-plant-specific microtubule nucleation factor MACET4 Sharol Schmidt and Andrei Smertenko* ABSTRACT that the diameter of the cell, continuous microtubule polymerization, Here, we show that the embryophyte (land-plant)-specific protein de-polymerization and re-polymerization drives phragmoplast MACERATOR4 (MACET4) binds microtubules in vitro and in vivo, expansion towards the cortical division zone located at the plasma promotes microtubule polymerization at sub-critical tubulin membrane of the cell. Attachment of the cell plate to a site within the concentrations, decreases the lag phase in microtubule bulk cortical division zone leads to phragmoplast disassembly. polymerization assays, and colocalizes with microtubule nucleation Phragmoplast microtubules are twice as dynamic as interphase sites. Furthermore, we find that MACET4 forms oligomers that induce microtubules (Hush et al., 1994; Smertenko et al., 2011). One aster formation in vitro in a manner that is similar to aster formation of the underlying reasons for this difference is continuous mediated by centrosomes and TPX2. MACET4 is expressed during cell depolymerization of microtubules in the region where cell plate division and accumulates at the microtubule nucleation regions of the synthesis is accomplished and polymerization of microtubules on plant-specific cytokinetic microtubule array, the phragmoplast. We the phragmoplast outer edge. What drives the depolymerization found that MACET4 localizes to the preprophase band and the cortical remains unknown, but new microtubules in the phragmoplast are γ γ division zone, but not the spindle. MACET4 appears as cytoplasmic foci nucleated by the -tubulin ring complex ( -TuRC; Hashimoto, in vivo and forms octamers in vitro. -

Role of Phragmoplast Filaments in Cell-Plate Formation
•7. Cell Sci. i, 455-462 (1966) 45 5 Printed in Great Britain ROLE OF PHRAGMOPLAST FILAMENTS IN CELL-PLATE FORMATION A. BAJER Department of Biology, University of Oregon ANDR. D.ALLEN* Department of Biology, Princeton University SUMMARY With the differential interference microscope (Nomarski system), it is possible to observe the interaction of filaments of the phragmoplast with micron-size retractile particles and vesicles, very close to the resolution limit of the microscope, during formation of the cell plate. Filaments occupying the birefringent zone of the phragmoplast (i.e. remnants of the continuous and interzonal fibres of the mitotic spindle) shorten and thicken in their mid-region to become spindle-shaped. The thickenings later fuse laterally to form the cell plate. During the process of thickening, vesicles can be seen to move toward the plate in close association with the filaments on both sides of the phragmoplast. These observations, taken together with informa- tion from electron micrographs of thin sections of Haemanthus endosperm and other dividing cells, confirm the notion that the fibrillar component of the phragmoplast has a transport function. The sizes of particles entering the phragmoplast region by saltation suggest that they correspond to Golgi bodies, which have been suspected of producing vesicles that contribute to cell-plate formation. Neither light nor electron micrographs have yet provided any insight into the mechanism of transport of these vesicles. INTRODUCTION Cytokinesis in plant cells typically entails the formation of the phragmoplast, cell plate and cell wall. The phragmoplast is a differentiated region of the plant cell which forms in late anaphase or early telophase of division between the separating groups of sister chromosomes. -

Arabidopsis TANGLED Identifies the Division Plane Throughout Mitosis and Cytokinesis
UC San Diego UC San Diego Previously Published Works Title Arabidopsis TANGLED identifies the division plane throughout mitosis and cytokinesis Permalink https://escholarship.org/uc/item/15g4b2w7 Journal Current Biology, 17(21) ISSN 0960-9822 Authors Walker, Keely L Müller, Sabine Moss, Dorianne et al. Publication Date 2007-11-01 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Current Biology 17, 1827–1836, November 6, 2007 ª2007 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2007.09.063 Article Arabidopsis TANGLED Identifies the Division Plane throughout Mitosis and Cytokinesis Keely L. Walker,1,3 Sabine Mu¨ ller,1,2,4 Dorianne Moss,2 are permanently established when daughter cells are David W. Ehrhardt,2 and Laurie G. Smith1,* formed at cytokinesis. Consequently, proper orientation 1Section of Cell and Developmental Biology of division planes during development is critical for the University of California, San Diego cellular organization of plant tissues. Unlike most other 9500 Gilman Drive eukaryotic cells, the division planes of plant cells are es- La Jolla, California 92093-0116 tablished prior to mitosis. During S or G2 phase of the 2Carnegie Institution cell cycle, most plant cells form a cortical ring of micro- Department of Plant Biology tubules and F-actin called the preprophase band (PPB) 260 Panama Street at the future division plane as the premitotic nucleus mi- Stanford, California 94305 grates into this plane [1]. The PPB persists throughout prophase, but is disassembled upon nuclear-envelope breakdown as the mitotic spindle forms [1, 2]. During Summary cytokinesis, a new cell wall (cell plate) is initiated through the action of the phragmoplast, another F-actin- Background: In premitotic plant cells, the future division and microtubule-based structure. -

Cytokinesis-Salem2003.Pdf
Cytokinesis = 1412 words Field of study: Classical transmission genetics, developmental genetics Significance: Cytokinesis is one of the most significant events that occur during the last phase of cell divisions. Some distinct features exist in cytokinesis of microbes, animal and plant cells. The partitioning of cytoplasm during meiosis and related sexual reproduction also act to determine the fate of resulted daughter cells. Key terms binary fission: the type of cell division in prokaryote by which the plasma membrane and cell wall grow inward and divide the cell in two cytokinesis: follows mitosis; a process that divides the entire cell into two new cells interphase: precedes mitosis in cell cycle; a period of intense cellular activities that include the DNA replication mitosis: nuclear division, a process of allotting a complete set of chromosomes to two daughter nuclei meiosis: a type of cell division that leads to production of gametes (e.g. sperm and egg) during sexual reproduction the cell cycle: a regular and repeated sequence of events pass through by dividing cells Events Leading to Cytokinesis Cytokinesis is the division or partitioning of the cytoplasm following the equal division of genetic material into the daughter cells. Before a given cell can divide, its genetic material – deoxyribonucleic acid or DNA has to be duplicated through DNA replication. The identical copies of DNA are then separated into one of the two daughter cells through a multiple step process, of which details vary among prokaryotes, plants and animals. With a single chromosome and no nucleus, prokaryotes (such as bacteria) utilize a simple method of cell division called binary fission (meaning “splitting in two”). -

Glossary.Pdf
Glossary Pronunciation Key accessory fruit A fruit, or assemblage of fruits, adaptation Inherited characteristic of an organ- Pronounce in which the fleshy parts are derived largely or ism that enhances its survival and reproduc- a- as in ace entirely from tissues other than the ovary. tion in a specific environment. – Glossary Ј Ј a/ah ash acclimatization (uh-klı¯ -muh-tı¯-za -shun) adaptive immunity A vertebrate-specific Physiological adjustment to a change in an defense that is mediated by B lymphocytes ch chose environmental factor. (B cells) and T lymphocytes (T cells). It e¯ meet acetyl CoA Acetyl coenzyme A; the entry com- exhibits specificity, memory, and self-nonself e/eh bet pound for the citric acid cycle in cellular respi- recognition. Also called acquired immunity. g game ration, formed from a fragment of pyruvate adaptive radiation Period of evolutionary change ı¯ ice attached to a coenzyme. in which groups of organisms form many new i hit acetylcholine (asЈ-uh-til-ko–Ј-le¯n) One of the species whose adaptations allow them to fill dif- ks box most common neurotransmitters; functions by ferent ecological roles in their communities. kw quick binding to receptors and altering the perme- addition rule A rule of probability stating that ng song ability of the postsynaptic membrane to specific the probability of any one of two or more mu- o- robe ions, either depolarizing or hyperpolarizing the tually exclusive events occurring can be deter- membrane. mined by adding their individual probabilities. o ox acid A substance that increases the hydrogen ion adenosine triphosphate See ATP (adenosine oy boy concentration of a solution.