Phragmoplast Microtubule Dynamics – a Game of Zones Andrei Smertenko1,2,‡, Seanna L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Role of the BUB3 Protein in Phragmoplast Microtubule Reorganization During Cytokinesis
UC Davis UC Davis Previously Published Works Title Publisher Correction: Role of the BUB3 protein in phragmoplast microtubule reorganization during cytokinesis. Permalink https://escholarship.org/uc/item/1gw1799k Journal Nature plants, 4(9) ISSN 2055-0278 Authors Zhang, Hongchang Deng, Xingguang Sun, Baojuan et al. Publication Date 2018-09-01 DOI 10.1038/s41477-018-0215-9 Peer reviewed eScholarship.org Powered by the California Digital Library University of California ARTICLES https://doi.org/10.1038/s41477-018-0192-z Corrected: Publisher Correction Role of the BUB3 protein in phragmoplast microtubule reorganization during cytokinesis Hongchang Zhang1,2,6, Xingguang Deng2,3,6, Baojuan Sun2,4, Sonny Lee Van2, Zhensheng Kang5, Honghui Lin3, Yuh-Ru Julie Lee 2* and Bo Liu 2* The evolutionarily conserved WD40 protein budding uninhibited by benzimidazole 3 (BUB3) is known for its function in spindle assembly checkpoint control. In the model plant Arabidopsis thaliana, nearly identical BUB3;1 and BUB3;2 proteins decorated the phragmoplast midline through interaction with the microtubule-associated protein MAP65-3 during cytokinesis. BUB3;1 and BUB3;2 interacted with the carboxy-terminal segment of MAP65-3 (but not MAP65-1), which harbours its microtubule- binding domain for its post-mitotic localization. Reciprocally, BUB3;1 and BUB3;2 also regulated MAP65-3 localization in the phragmoplast by enhancing its microtubule association. In the bub3;1 bub3;2 double mutant, MAP65-3 localization was often dissipated away from the phragmoplast midline and abolished upon treatment of low doses of the cytokinesis inhibitory drug caffeine that were tolerated by the control plant. The phragmoplast microtubule array exhibited uncoordinated expansion pat- tern in the double mutant cells as the phragmoplast edge reached the parental plasma membrane at different times in differ- ent areas. -

The Revised Classification of Eukaryotes
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/231610049 The Revised Classification of Eukaryotes Article in Journal of Eukaryotic Microbiology · September 2012 DOI: 10.1111/j.1550-7408.2012.00644.x · Source: PubMed CITATIONS READS 961 2,825 25 authors, including: Sina M Adl Alastair Simpson University of Saskatchewan Dalhousie University 118 PUBLICATIONS 8,522 CITATIONS 264 PUBLICATIONS 10,739 CITATIONS SEE PROFILE SEE PROFILE Christopher E Lane David Bass University of Rhode Island Natural History Museum, London 82 PUBLICATIONS 6,233 CITATIONS 464 PUBLICATIONS 7,765 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Biodiversity and ecology of soil taste amoeba View project Predator control of diversity View project All content following this page was uploaded by Smirnov Alexey on 25 October 2017. The user has requested enhancement of the downloaded file. The Journal of Published by the International Society of Eukaryotic Microbiology Protistologists J. Eukaryot. Microbiol., 59(5), 2012 pp. 429–493 © 2012 The Author(s) Journal of Eukaryotic Microbiology © 2012 International Society of Protistologists DOI: 10.1111/j.1550-7408.2012.00644.x The Revised Classification of Eukaryotes SINA M. ADL,a,b ALASTAIR G. B. SIMPSON,b CHRISTOPHER E. LANE,c JULIUS LUKESˇ,d DAVID BASS,e SAMUEL S. BOWSER,f MATTHEW W. BROWN,g FABIEN BURKI,h MICAH DUNTHORN,i VLADIMIR HAMPL,j AARON HEISS,b MONA HOPPENRATH,k ENRIQUE LARA,l LINE LE GALL,m DENIS H. LYNN,n,1 HILARY MCMANUS,o EDWARD A. D. -

Molecular Biology and Applied Genetics
MOLECULAR BIOLOGY AND APPLIED GENETICS FOR Medical Laboratory Technology Students Upgraded Lecture Note Series Mohammed Awole Adem Jimma University MOLECULAR BIOLOGY AND APPLIED GENETICS For Medical Laboratory Technician Students Lecture Note Series Mohammed Awole Adem Upgraded - 2006 In collaboration with The Carter Center (EPHTI) and The Federal Democratic Republic of Ethiopia Ministry of Education and Ministry of Health Jimma University PREFACE The problem faced today in the learning and teaching of Applied Genetics and Molecular Biology for laboratory technologists in universities, colleges andhealth institutions primarily from the unavailability of textbooks that focus on the needs of Ethiopian students. This lecture note has been prepared with the primary aim of alleviating the problems encountered in the teaching of Medical Applied Genetics and Molecular Biology course and in minimizing discrepancies prevailing among the different teaching and training health institutions. It can also be used in teaching any introductory course on medical Applied Genetics and Molecular Biology and as a reference material. This lecture note is specifically designed for medical laboratory technologists, and includes only those areas of molecular cell biology and Applied Genetics relevant to degree-level understanding of modern laboratory technology. Since genetics is prerequisite course to molecular biology, the lecture note starts with Genetics i followed by Molecular Biology. It provides students with molecular background to enable them to understand and critically analyze recent advances in laboratory sciences. Finally, it contains a glossary, which summarizes important terminologies used in the text. Each chapter begins by specific learning objectives and at the end of each chapter review questions are also included. -

Formation and Structure of Filamentous Systems for Insect Flight and Mitotic Movements Melvyn Dennis Goode Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1967 Formation and structure of filamentous systems for insect flight and mitotic movements Melvyn Dennis Goode Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Genetics Commons Recommended Citation Goode, Melvyn Dennis, "Formation and structure of filamentous systems for insect flight and mitotic movements " (1967). Retrospective Theses and Dissertations. 3391. https://lib.dr.iastate.edu/rtd/3391 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. FORMATION AND STRUCTURE OF FILAMENTOUS SYSTEMS FOR INSECT FLIGHT AND MITOTIC MOVEMENTS by Melvyn Dennis Goode A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Cell Biology Approved: Signature was redacted for privacy. In Charge oi Major Work Signature was redacted for privacy. Chairman Advisory Committee Cell Biology Program Signature was redacted for privacy. Signature was redacted for privacy. Iowa State University Of Science and Technology Ames, Iowa: 1967 ii TABLE OF CONTENTS Page I. INTRODUCTION 1 PART ONE. THE MITOTIC APPARATUS OF A GIANT AMEBA 5 II. THE STRUCTURE AND PROPERTIES OF THE MITOTIC APPARATUS 6 A. Introduction .6 1. Early studies of mitosis 6 2. The mitotic spindle in living cells 7 3. -

Functions of the Arabidopsis Kinesin Superfamily of Microtubule-Based Motor Proteins Chuanmei Zhu
Washington University in St. Louis Washington University Open Scholarship Biology Faculty Publications & Presentations Biology 10-2012 Functions of the Arabidopsis kinesin superfamily of microtubule-based motor proteins Chuanmei Zhu Ram Dixit Washington University in St Louis, [email protected] Follow this and additional works at: https://openscholarship.wustl.edu/bio_facpubs Part of the Biochemistry Commons, Biology Commons, and the Plant Biology Commons Recommended Citation Zhu, Chuanmei and Dixit, Ram, "Functions of the Arabidopsis kinesin superfamily of microtubule-based motor proteins" (2012). Biology Faculty Publications & Presentations. 79. https://openscholarship.wustl.edu/bio_facpubs/79 This Article is brought to you for free and open access by the Biology at Washington University Open Scholarship. It has been accepted for inclusion in Biology Faculty Publications & Presentations by an authorized administrator of Washington University Open Scholarship. For more information, please contact [email protected]. Functions of the Arabidopsis kinesin superfamily of microtubule-based motor proteins Chuanmei Zhu and Ram Dixit Biology Department, Washington University, St. Louis, MO 63130 Corresponding author Ram Dixit 1 Brookings Drive, CB 1137 St. Louis, MO 63130. Phone: (314) 935-8823 Fax: (314) 935-4432 Email: [email protected] Keywords Plant, cortical microtubule, preprophase band, spindle, phragmoplast ABSTRACT Plants possess a large number of microtubule-based kinesin motor proteins. While the Kinesin-2, 3, 9 and 11 families are absent from land plants, the Kinesin-7 and 14 families are greatly expanded. In addition, some kinesins are specifically present only in land plants. The distinctive inventory of plant kinesins suggests that kinesins have evolved to perform specialized functions in plants. -

Rangap1 Is a Continuous Marker of the Arabidopsis Cell Division Plane
RanGAP1 is a continuous marker of the Arabidopsis cell division plane Xianfeng Morgan Xua,1, Qiao Zhaoa,2, Thushani Rodrigo-Peirisa, Jelena Brkljacica, Chao Sylvia Hea,1, Sabine Mu¨ llerb, and Iris Meiera,3 aPlant Biotechnology Center and Department of Plant Cellular and Molecular Biology, The Ohio State University, Columbus, OH 43210; and bSchool of Biological Sciences, University of Auckland, Auckland 1142, New Zealand Edited by Maarten J. Chrispeels, University of California at San Diego, La Jolla, CA, and approved October 8, 2008 (received for review June 30, 2008) In higher plants, the plane of cell division is faithfully predicted by were found to interact with TANGLED and a pok1 pok2 double the preprophase band (PPB). The PPB, a cortical ring of microtu- mutant resembles the maize tan mutant in terms of misoriented bules and F-actin, disassembles upon nuclear-envelope break- division planes (9). Although the data suggest a role for kinesins down. During cytokinesis, the expanding cell plate fuses with the and the pioneer protein TANGLED in division-plane definition, plasma membrane at the cortical division site, the site of the former the molecular mechanism of the process remains unknown. PPB. The nature of the ‘‘molecular memory’’ that is left behind by Ran is a small GTPase that in vertebrates controls multiple the PPB and is proposed to guide the cell plate to the cortical cellular processes including nucleocytoplasmic transport, spindle division site is unknown. RanGAP is the GTPase activating protein assembly, nuclear envelope reassembly, centrosome duplication, of the small GTPase Ran, which provides spatial information for and cell-cycle control (ref. -
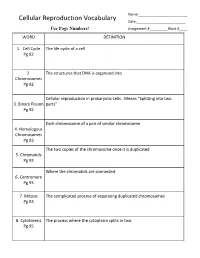
Cellular Reproduction Vocabulary Date:______Use Page Numbers! Assignment #______Block #____ WORD DEFINITION
Name:_________________________ Cellular Reproduction Vocabulary Date:_________________________ Use Page Numbers! Assignment #_________ Block #____ WORD DEFINITION 1. Cell Cycle The life cycle of a cell Pg 92 2. The structures that DNA is organized into Chromosomes Pg 92 Cellular reproduction in prokaryotic cells. Means “Splitting into two 3. Binary Fission parts” Pg 92 Each chromosome of a pair of similar chromosome 4. Homologous Chromosomes Pg 93 The two copies of the chromosome once it is duplicated 5. Chromatids Pg 93 Where the chromatids are connected 6. Centromere Pg 93 7. Mitosis The complicated process of separating duplicated chromosomes Pg 93 8. Cytokinesis The process where the cytoplasm splits in two Pg 95 WORD DEFINITION 9. Budding A type of asexual reproduction, where a piece of the parents body Pg 612 develops into an independent organism 10. A type of asexual reproduction, where the organism breaks into two or Regeneration more parts, each growing into a new organism that is genetically identical (fragmentation) to the parent Pg 612 The production of offspring by combining the genetic material of more 11. Sexual than one parent reproduction Pg 613 A single parent has offspring that are identical to itself 12. Asexual reproduction Pg 613 The male sex cell 13. Sperm Pg 613 The female sex cell 14. Egg Pg 613 The new type of cell that is made when an egg’s nucleus fuses with a 15. Zygote sperm’s nucleus Pg 613 16. Spores Small reproductive cells that are protected by a thick cell wall Pg 256 WORD DEFINITION 17. Sex Cells Specialized cells that combine to form a zygote, they have half the normal Pg 114 number of chromosomes, one of each pair. -

Kinetochore Protein Depletion Underlies Cytokinesis Failure And
RESEARCH ARTICLE Kinetochore protein depletion underlies cytokinesis failure and somatic polyploidization in the moss Physcomitrella patens Elena Kozgunova1*, Momoko Nishina2, Gohta Goshima2* 1International Collaborative Programme in Science, Graduate School of Science, Nagoya University, Nagoya, Japan; 2Division of Biological Science, Graduate School of Science, Nagoya University, Nagoya, Japan Abstract Lagging chromosome is a hallmark of aneuploidy arising from errors in the kinetochore–spindle attachment in animal cells. However, kinetochore components and cellular phenotypes associated with kinetochore dysfunction are much less explored in plants. Here, we carried out a comprehensive characterization of conserved kinetochore components in the moss Physcomitrella patens and uncovered a distinct scenario in plant cells regarding both the localization and cellular impact of the kinetochore proteins. Most surprisingly, knock-down of several kinetochore proteins led to polyploidy, not aneuploidy, through cytokinesis failure in >90% of the cells that exhibited lagging chromosomes for several minutes or longer. The resultant cells, containing two or more nuclei, proceeded to the next cell cycle and eventually developed into polyploid plants. As lagging chromosomes have been observed in various plant species in the wild, our observation raised a possibility that they could be one of the natural pathways to polyploidy in plants. DOI: https://doi.org/10.7554/eLife.43652.001 *For correspondence: [email protected] (EK); [email protected] (GG) Competing interests: The Introduction authors declare that no The kinetochore is a macromolecular complex that connects chromosomes to spindle microtubules competing interests exist. and plays a central role in chromosome segregation. Kinetochore malfunction causes checkpoint- Funding: See page 14 dependent mitotic arrest, apoptosis, and/or aneuploidy-inducing chromosome missegregation (Potapova and Gorbsky, 2017). -

Electron Microscope Studies on the Mitotic Figure II . Phragmoplast and Cell Plate1
98 Cytologia 24 Electron Microscope Studies on the Mitotic Figure II . Phragmoplast and cell plate1 Syoiti Sato BotanicalInstitute, Faculty of Science,University of Tokyo, Tokyo,Japan ReceivedDecember 18, 1958 The fine structure of the metaphase spindle, in his first report of "electron microscope studies on the mitotic figure" , has been already described by the present author (1958). In the present paper, both the phragmoplast appearing at the end of anaphase and completed in telophase of plant mitotic cells, and also the cell plate arising in the equatorial zone of the phrag moplast, are by the help of the electron microscope described with reference to their fine structures, and also with reference to the origin and the develop ment of both apparatus. The hypotheses on the formation of the cell wall are also discussed. Material and methods The material used was the pollen mother cell of Lilium lancifolium Thunb. It was fixed in CdCl2 solution used by Wada and Fukunaga (1957). This method was already described by the present author in a previous paper (1958). The procedure of preparation of samples was as follows: pollen mother cells, in either anaphase or telophase stage, were fixed in an equivalent mixture of 10-1M CdCl2 solution and 100 per cent alcohol for 30 minutes. Later, the materials were dehydrated through the alcohol series, and were embedded in 7 parts n-buthyl methacrylate and 3 parts methyl methacrylate and polymerized at 45•Ž. Sections were cut with the Hitachi Ultra-Microtome. The electron microscope used, was the Hitachi Type HU-10. Results and discussion The phragmoplast The fine structure of the metaphase spindle which has been described by the present author in a former paper (1958), is considered to be sub stantially common to both plant and animal cells. -

KCH Kinesin Drives Nuclear Transport and Cytoskeletal Coalescence for Tip Cell Growth
bioRxiv preprint doi: https://doi.org/10.1101/308775; this version posted April 26, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. KCH kinesin drives nuclear transport and cytoskeletal coalescence for tip cell growth Moé Yamada and Gohta Goshima# Division of Biological Science, Graduate School of Science, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8602, Japan #[email protected] Long-distance transport along including organelles, proteins, and RNA, microtubules (MTs) is critical for are transported to their appropriate intracellular organisation. In animals, positions where they specifically function in antagonistic motor proteins kinesin response to internal and external signals. (plus end-directed) and dynein (minus Although it had been believed that plants end-directed) drive cargo transport. In predominantly utilize actin and myosin to land plants, however, the identity of move cellular components, recent studies motors responsible for transport is have uncovered the prevalence of poorly understood, as genes encoding microtubule (MT)-dependent transport as cytoplasmic dynein are missing. How well (Kong et al., 2015; Miki et al., 2015; other functions of dynein are brought Nakaoka et al., 2015; Zhu et al., 2015; about in plants also remains unknown. Yamada et al., 2017). However, a unique Here, we show that a subclass of the feature of plant motor systems is that the kinesin-14 family, KCH—which can also genes encoding cytoplasmic dynein, the bind actin—drives MT minus sole MT minus end-directed transporter in end-directed nuclear transport in the animals, have been lost during plant moss Physcomitrella patens. -

A Short History of Plant Light Microscopy
1 From the identification of ‘Cells’, to Schleiden & Schwann’s Cell Theory, to Confocal 2 Microscopy and GFP lighting up the Plant Cytoskeleton, to Super-Resolution 3 Microscopy with Single Molecule Tracking: Here’s... 4 A Short History of Plant Science Chapter 5: 5 A Short History of Plant Light Microscopy 6 Marc Somssich 7 School of BioSciences, the University of Melbourne, Parkville 3010, VIC, Australia 8 Email: [email protected] ; Twitter: @somssichm 9 http://dx.doi.org/10.5281/zenodo.4682573 10 When the microscope was first introduced to scientists in the 17th century it started a 11 revolution. Suddenly a whole new world, invisible to the naked eye, opened up to curious 12 explorers. In response to this realization Nehemiah Grew, one of the early microscopists, 13 noted in 1682 ‘that Nothing hereof remains further to be known, is a Thought not well 14 Calculated.’1. And indeed, with ever increasing resolution, there really does not seem to be an 15 end to what can be explored with a microscope. 16 The Beginnings: Plant Internal Structures and ‘Cells’ (1600-1835) 17 While simple lenses were being used as magnifying glasses for several centuries, the early 18 17th century brought the invention of the compound microscope, and with it launched the 19 scientific field of microscopy2. It is not clear who invented the first microscope, but it was 20 most likely developed from early telescopes2. Galileo Galilei built his first telescope in the 21 early 1600s and used it to chart the stars2. He subsequently published his treatise ‘Sidereus 22 nuncius’ (1610) about his observations2,3. -

Marine Botany Midterm 2014 Name:______
Marine Botany Midterm 2014 Name:_________________________ Compare and Contrast the following items (20pts): 1) spore vs gamete Spore: unicellular, must settle & grow, product of mitosis(Mt) or meiosis (Me) Gamete: unicellular, must fuse or die, product of mitosis(Mt) or meiosis (Me) 2) monoecious vs dioecious Dioecious- having the male & female reproductive structure borne on separate individual plants; said of the species -two houses Monoecious- having the male & female reproductive structure borne on the same individual plants -one house 3) phycoplast vs. phragmoplast Phycoplast: microtubules parallel to dividing plane -rare in algae Phragmoplast: double microtubules perpendicular to dividing plane-common in algae & land plants 4) haplontic vs diplontic Haplontic: 1N thallus, the zygote is the only diploid stage Diplontic: 2N thallus, the gametes are the only haploid stage 5) parenchymatous vs. coenocytic thallus construction Parenchyma – undifferentiated, isodiometric cells generated by a meristem Cells division in any plane , not filamentous Coenocytic – thallus made up of filaments, multi-nucleate, lacking crosswalls, siphonous 1 Marine Botany Midterm 2014 Name:_________________________ Match with the correct Division: Chlorophyta, Heterokontophyta, both, or neither (12 points) a. Plantae __Chloro__________________ b. Chlorophyll A _Both___________________ c. Flowers _______Neither_____________ d. Phycobilins _____Neither_______________ e. Amylose ___Chloro_________ f. Thylakoids in stacks of 2-6 _________Chloro___ g. Bacteria ______Neither____________ h. Flagella ____Both_____________ i. Haplontic life history _____Chloro_________ j. 2 endosymobiotic event ____Hetero__________ k. Chromalvaeolates ______Hetero_________ l. Mannitol___________Hetero___________ Match the following Classes or Orders to the appropriate characteristic, term, or genus. Each term will be used only once. (10 points) Cladophorales ___C_____ A. parenchymatous thallus Ulotricales ___J_____ B. clockwise basal body orientation Chlorophyceae _____B____ C.