Nanoporous Glass-Ceramics Transparent in Infrared Range to Be Used As Optical Sensor-Mechanical and Viscoelastic Properties of the TAS (Te-As-Se) Glass
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ocean Drilling Program Initial Reports Volume
Sigurdsson, H., Leckie, R.M., Acton, G.D., et al., 1997 Proceedings of the Ocean Drilling Program, Initial Reports, Vol. 165 2. EXPLANATORY NOTES1 Shipboard Scientific Party2 INTRODUCTION Shipboard Scientific Procedures Numbering of Sites, Holes, Cores, and Samples In this chapter, we have assembled information that documents our scientific methods. This information concerns only shipboard Drilling sites are numbered consecutively from the first site methods described in the site reports in the Initial Reports volume of drilled by the Glomar Challenger in 1968. A site number refers to the Leg 165 Proceedings of the Ocean Drilling Program (ODP). one or more holes drilled while the ship was positioned over one Methods for shore-based analyses of Leg 165 data will be described acoustic beacon. Multiple holes may be drilled at a single site by pull- in the individual scientific contributions to be published in the Scien- ing the drill pipe above the seafloor (out of the hole), moving the ship tific Results volume. some distance from the previous hole, and then drilling another hole. Coring techniques and core handling, including the numbering of In some cases, the ship may return to a previously occupied site to sites, holes, cores, sections, and samples were the same as those re- drill additional holes. ported in previous Initial Reports volumes of the Proceedings of the For all ODP drill sites, a letter suffix distinguishes each hole Ocean Drilling Program with two exceptions: The core sections drilled at the same site. For example, the first hole drilled is assigned from Holes 1002D and 1002E in the Cariaco Basin were not split dur- the site number modified by the suffix "A," the second hole takes the ing Leg 165; instead, they were transported to the Gulf Coast Repos- site number and suffix "B," and so forth. -

Photonic Glass-Ceramics: Consolidated Outcomes and Prospects Brigitte Boulard1, Tran T
Photonic glass-ceramics: consolidated outcomes and prospects Brigitte Boulard1, Tran T. T. Van2, Anna Łukowiak3, Adel Bouajaj4, Rogéria Rocha Gonçalves5, Andrea Chiappini6, Alessandro Chiasera6, Wilfried Blanc7, Alicia Duran8, Sylvia Turrell9, Francesco Prudenzano10, Francesco Scotognella11, Roberta Ramponi11, Marian Marciniak12, Giancarlo C. Righini13,14, Maurizio Ferrari6,13,* 1 Institut des Molécules et Matériaux du Mans, UMR 6283, Equipe Fluorures, Université du Maine, Av. Olivier Messiaen, 72085 Le Mans cedex 09, France. 2 University of Science Ho Chi Minh City, 227 Nguyen Van Cu, Dist.5, HCM Vietnam. 3 Institute of Low Temperature and Structure Research, PAS, ul. Okolna 2, 50-950 Wroclaw, Poland. 4 Laboratory of innovative technologies, LTI, ENSA–Tangier, University Abdelmalek Essaâdi, Tangier, Morocco. 5 Departamento de Química, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo - Av. Bandeirantes, 3900, CEP 14040-901, Ribeirão Preto/SP, Brazil 6 CNR-IFN, CSMFO Lab., Via alla Cascata 56/c, Povo, 38123 Trento, Italy. 7 Université Nice Sophia Antipolis, CNRS LPMC, UMR 7336, 06100 Nice, France. 8 Instituto de Ceramica y Vidrio (CSIC), C/Kelsen 5, Campus de Cantoblanco, 28049 Madrid, Spain. 9 LASIR (CNRS, UMR 8516) and CERLA, Université Lille 1, 59650 Villeneuve d’Ascq, France. 10 Politecnico di Bari, DEI, Via E. Orabona 4, Bari, 70125, Italy. 11 IFN-CNR and Department of Physics, Politecnico di Milano, p.zza Leonardo da Vinci 32, 20133 Milano, Italy 12 National Institute of Telecommunications, 1 Szachowa Street, 04 894 Warsaw, Poland. 13 Centro di Studi e Ricerche “Enrico Fermi”, Piazza del Viminale 2, 00184 Roma, Italy. 14 MipLAB. IFAC - CNR, Via Madonna del Piano 10, 50019 Sesto Fiorentino, Italy. -

The American Ceramic Society 25Th International Congress On
The American Ceramic Society 25th International Congress on Glass (ICG 2019) ABSTRACT BOOK June 9–14, 2019 Boston, Massachusetts USA Introduction This volume contains abstracts for over 900 presentations during the 2019 Conference on International Commission on Glass Meeting (ICG 2019) in Boston, Massachusetts. The abstracts are reproduced as submitted by authors, a format that provides for longer, more detailed descriptions of papers. The American Ceramic Society accepts no responsibility for the content or quality of the abstract content. Abstracts are arranged by day, then by symposium and session title. An Author Index appears at the back of this book. The Meeting Guide contains locations of sessions with times, titles and authors of papers, but not presentation abstracts. How to Use the Abstract Book Refer to the Table of Contents to determine page numbers on which specific session abstracts begin. At the beginning of each session are headings that list session title, location and session chair. Starting times for presentations and paper numbers precede each paper title. The Author Index lists each author and the page number on which their abstract can be found. Copyright © 2019 The American Ceramic Society (www.ceramics.org). All rights reserved. MEETING REGULATIONS The American Ceramic Society is a nonprofit scientific organization that facilitates whether in print, electronic or other media, including The American Ceramic Society’s the exchange of knowledge meetings and publication of papers for future reference. website. By participating in the conference, you grant The American Ceramic Society The Society owns and retains full right to control its publications and its meetings. -

Celebrating 100 Years
AMERICANa CERAMICting SOCIETY ars Celebr 100 ye bullemerginge ceramicstin & glass technology SEPTEMBER 2021 Laser-driven chemical vapor deposition for high-performance fibers and powders New issue inside: SEPTEMBER 2021 • VOLUME 2 • ISSUE 3 www.ceramics.org/ceramicandglassmanufacturing THE VALUE OF COLLABORATION: PARTNERSHIPS ARE A PATH TO SUCCESS ABET ENSURES QUALITY IN UNIVERSITY ENGINEERING EDUCATION ACerS Awards of 2021 | Coe College glass research | Big science in aerospace When it Comes to Heat, We Sweat the Details! Your firing needs are unique. Our laboratory can run tests to So why use an “off the shelf” help identify your process kiln in your process? boundaries. Through our toll firing facility, we can At Harrop, we get it. help to further define That’s why, for over a the equipment/ century, we’ve been processing putting in the hard work combination that to design and service works best for your custom kilns. Is it harder material. And if you to do things this way? are not ready for a Yes. Is the extra effort new kiln, we can toll worth it? You bet! fire your material to help meet your At Harrop, we don’t production needs. stop there. If you aren’t sure what you Does your current need, we can help. kiln company sweat the details? www.harropusa.com 1.614.231.3621 Harrop Ad Sweat the Details ACerS Full Size w 100 logo.indd 1 5/21/20 9:33 AM contents September 2021 • Vol. 100 No.7 feature articles department Announcing ACerS Awards of 2021 News & Trends . 3 29 The Society will honor members and corporations at the Spotlight . -

Lecture #16 Glass-Ceramics: Nature, Properties and Processing Edgar Dutra Zanotto Federal University of São Carlos, Brazil [email protected] Spring 2015
Glass Processing Lecture #16 Glass-ceramics: Nature, properties and processing Edgar Dutra Zanotto Federal University of São Carlos, Brazil [email protected] Spring 2015 Lectures available at: www.lehigh.edu/imi Sponsored by US National Science Foundation (DMR-0844014) 1 Glass-ceramics: nature, applications and processing (2.5 h) 1- High temperature reactions, melting, homogeneization and fining 2- Glass forming: previous lectures 3- Glass-ceramics: definition & applications (March 19) Today, March 24: 4- Composition and properties - examples 5- Thermal treatments – Sintering (of glass powder compactd) or -Controlled nucleation and growth in the glass bulk 6- Micro and nano structure development April 16 7- Sophisticated processing techniques 8- GC types and applications 9- Concluding remmarks 2 Review of Lecture 15 Glass-ceramics -Definition -History -Nature, main characteristics -Statistics on papers / patents - Properties, thermal treatments micro/ nanostructure design 3 Reading assignments E. D. Zanotto – Am. Ceram. Soc. Bull., October 2010 Zanotto 4 The discovery of GC Natural glass-ceramics, such as some types of obsidian “always” existed. René F. Réaumur – 1739 “porcelain” experiments… In 1953, Stanley D. Stookey, then a young researcher at Corning Glass Works, USA, made a serendipitous discovery ...… 5 <rms> 1nm Zanotto 6 Transparent GC for domestic uses Zanotto 7 Company Products Crystal type Applications Photosensitive and etched patterned Foturan® Lithium-silicate materials SCHOTT, Zerodur® β-quartz ss Telescope mirrors Germany -
Bowling Green Alumni Association Announces
THE AREA’S ONLY LOCALLY-OWNED & OPERATED NEWSPAPER | EST. OCTOBER 1, 1996 HE EOPLE S RIBUNE TNEWS FOR PIKEP, EASTERN AUDRAIN’& NORTHERNT LINCOLN COUNTIES FREE Published Every Tuesday • Vol. 26 - No. 42 • Tuesday, Aug. 3, 2021 • Online at www.thepeoplestribune.com Bowling Green Alumni Association Announces BanquetBY BRICE Speaker,CHANDLER EntertainmentLynyrd Skynyrd, The Allman Broth- STAFF WRITER ers, The Dave Matthews Band, and First held in 1985, the Bowling more. Green Alumni Association hosts its According to his bio, “Powell's annual alumni banquet each fall to work has been included on multiple honor graduating classes of the past gold and platinum records with nine and celebrate the education and different Grammy winning proj- memories of those important years ects.” at Bowling Green High School. Not only has he worked on such The organization also updates notable projects, but Powell has also members on one of its founding pur- cut vinyl records for the last 13- poses – the status of scholarships years with the Sam Phillips Record- awarded each year to graduating ing Service and his own company, seniors. Take Out “To date, Vinyl. the association Powell met has awarded his wife of 28- more than years, Susan, $411,050 in during a scholarships,” recording ses- the group sion at Ardent stated in its re- Studios for a cent banquet new band registration called The form. “Includ- Mother Sta- ing eighteen tion. $1,000 schol- When not Hot Weather arships to in the studio, 2020 gradu- “he remains a ates and six- diehard Saint teen $1,000 Louis Cardi- Did Not Deter scholarships to nals fan.” 2021 gradu- Attendees ates.” of this year's To celebrate banquet will Pike County Fair the accom- also be treated plishment and camaraderie, the as- to entertainment from an alumni sociation invites special guest choir under the direction of retired speakers and entertainers for a night vocal music instructor, Jack Bibb. -

Practical Aspects and Implications of Interfaces in Glass-Ceramics
SCHOTT North America, Inc. Interfaces in Functional Materials Practical aspects and implications of interfaces in glass-ceramics Mark J. Davis SCHOTT North America, Inc. Outline Key questions to address Interfacial effects in glass-ceramics---a laundry list Glass-ceramics in general: SCHOTT commercial examples Commercial or near-commercial gc / interface examples Key questions: review SCHOTT North America, Inc. Interfaces in Functional Materials Key Questions (from H. Jain) What has been the role of interfaces in the development of emerging applications? With regard to applications, what aspects of interfaces are most important and why? What are the scientific issues that require basic understanding of interfaces in glass-ceramics? What is the relative importance of each? What properties of glass-ceramics hold promise for the future? SCHOTT North America, Inc. Interfaces in Functional Materials Practical Effects (Internal) Microstructural development surface energies and their impact on nucleation general glass stability; controlled vs. un-controlled crystallization (i.e., critical cooling rate in a commercial setting vs. academic…) Structural detailed nature of interface (e.g., “pristine”, microcracked…) crack blunting processes residual stresses, crystal clamping permeability Electrical Effective connectivity Resistive / capacitive behavior Optical scattering effects SCHOTT North America, Inc. Interfaces in Functional Materials Practical Effects (External) Joining (low-temperature) Hydrophilic vs. hydrophobic -
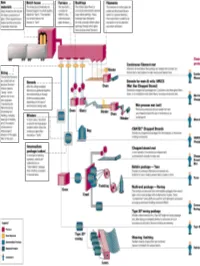
All About Fibers
RawRaw MaterialsMaterials ¾ More than half the mix is silica sand, the basic building block of any glass. ¾ Other ingredients are borates and trace amounts of specialty chemicals. Return © 2003, P. Joyce BatchBatch HouseHouse && FurnaceFurnace ¾ The materials are blended together in a bulk quantity, called the "batch." ¾ The blended mix is then fed into the furnace or "tank." ¾ The temperature is so high that the sand and other ingredients dissolve into molten glass. Return © 2003, P. Joyce BushingsBushings ¾The molten glass flows to numerous high heat-resistant platinum trays which have thousands of small, precisely drilled tubular openings, called "bushings." Return © 2003, P. Joyce FilamentsFilaments ¾This thin stream of molten glass is pulled and attenuated (drawn down) to a precise diameter, then quenched or cooled by air and water to fix this diameter and create a filament. Return © 2003, P. Joyce SizingSizing ¾The hair-like filaments are coated with an aqueous chemical mixture called a "sizing," which serves two main purposes: 1) protecting the filaments from each other during processing and handling, and 2) ensuring good adhesion of the glass fiber to the resin. Return © 2003, P. Joyce WindersWinders ¾ In most cases, the strand is wound onto high-speed winders which collect the continuous fiber glass into balls or "doffs.“ Single end roving ¾ Most of these packages are shipped directly to customers for such processes as pultrusion and filament winding. ¾ Doffs are heated in an oven to dry the chemical sizing. Return © 2003, P. Joyce IntermediateIntermediate PackagePackage ¾ In one type of winding operation, strands are collected into an "intermediate" package that is further processed in one of several ways. -

Stability of Materials for Use in Space-Based Interferometric Missions
STABILITY OF MATERIALS FOR USE IN SPACE-BASED INTERFEROMETRIC MISSIONS By ALIX PRESTON A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2010 1 °c 2010 Alix Preston 2 This is dedicated to all who were told they would fail, only to prove them wrong 3 ACKNOWLEDGMENTS Much of this work would not have been made possible if it were not for the help of many graduate and undergraduate students, faculty, and sta®. I would like to thank Ira Thorpe, Rachel Cruz, Vinzenz Vand, and Josep Sanjuan for their help and thoughtful discussions that were instrumental in understanding the nuances of my research. I would also like to thank Gabriel Boothe, Aaron Spector, Benjamin Balaban, Darsa Donelon, Kendall Ackley, and Scott Rager for their dedication and persistence to getting the job done. A special thanks is due for the physics machine shop, especially Marc Link and Bill Malphurs, who spent many hours on the countless projects I needed. Lastly, I would like to thank my advisor, Dr. Guido Mueller, who put up with me, guided me, and supported me in my research. 4 TABLE OF CONTENTS page ACKNOWLEDGMENTS ................................. 4 LIST OF TABLES ..................................... 9 LIST OF FIGURES .................................... 10 KEY TO ABBREVIATIONS ............................... 17 KEY TO SYMBOLS .................................... 19 ABSTRACT ........................................ 20 CHAPTER 1 INTRODUCTION .................................. 22 1.1 Space-Based Missions .............................. 23 1.2 GRACE ..................................... 23 1.3 GRACE Follow-On ............................... 25 1.4 LISA ....................................... 26 1.4.1 Introduction ............................... 26 1.4.2 Sources .................................. 27 1.4.2.1 Cosmological background sources ............. -

The Materials Science and Engineering of Advanced YB-Doped Glasses and Fibers for High-Power Lasers
Clemson University TigerPrints All Dissertations Dissertations May 2020 The Materials Science and Engineering of Advanced YB-Doped Glasses and Fibers for High-Power Lasers Thomas Wade Hawkins Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Recommended Citation Hawkins, Thomas Wade, "The Materials Science and Engineering of Advanced YB-Doped Glasses and Fibers for High-Power Lasers" (2020). All Dissertations. 2585. https://tigerprints.clemson.edu/all_dissertations/2585 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. THE MATERIALS SCIENCE AND ENGINEERING OF ADVANCED YB-DOPED GLASSES AND FIBERS FOR HIGH-POWER LASERS A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Materials Science and Engineering by Thomas Wade Hawkins May 2020 Accepted by: Dr. Liang Dong, Committee Chair Dr. John Ballato Dr. Peter Dragic Dr. Stephen Foulger Dr. Philip Brown ABSTRACT This research studies and yields new understandings into the materials science and engineering of advanced multicomponent glass systems, which is critical for next generation fiber lasers operating at high output powers. This begins with the study and development of Yb-doped glasses in the Al2O3-P2O5-SiO2 (APS) ternary system, fabricated using modified chemical vapor deposition (MCVD), that, despite being highly doped, possess an average refractive index matched to that of silica (SiO2). The highly doped active core material was subsequently processed through a multiple stack-and- draw process to realize a single fiber with high doping, compositionally-tailored index, and scalability for fiber lasers. -

Femtosecond Laser Direct-Write of Optofluidic Lab in Fiber Through Polymer-Coated Optical Fibers
Femtosecond Laser Direct-Write of Optofluidic Lab in Fiber through Polymer-Coated Optical Fibers by Kevin A. J. Joseph A thesis submitted in conformity with the requirements for the degree of Master of Applied Science Graduate Department of The Edward S. Rogers Sr. Department of Electrical and Computer Engineering University of Toronto Copyright c 2017 by Kevin A. J. Joseph Abstract Femtosecond Laser Direct-Write of Optofluidic Lab in Fiber through Polymer-Coated Optical Fibers Kevin A. J. Joseph Master of Applied Science Graduate Department of The Edward S. Rogers Sr. Department of Electrical and Computer Engineering University of Toronto 2017 Three-dimensional femtosecond laser processing of lab in fiber, the combination of integrated photonics and microfluidics inside optical fiber, was demonstrated in silica glass fibers coated with polymer buffer. This enables the laser fabrication of lab-on- chip functionalities onto optical fiber without the time-consuming and mechanically- compromising process of buffer removal. In this thesis, an assessment of laser-induced damage in buffer-coated fiber is reported, along with methods to avoid and mitigate this damage. Further, selective buffer machining is studied enabling in-tandem processing of the glass fiber core and cladding with the polymer buffer. Structuring of the fiber core, cladding photonic circuits, and microfluidics were demonstrated in buffer-coated fiber without removal of or damage to the polymer buffer. The methods and processes here make the lab in fiber platform to be more industry viable and opens new opportunities for device architectures spanning across the fiber core, cladding, and buffer, representing a significant technological advancement. -

Fiber Reinforcement of a Resin Modified Glass Ionomer Cement Abstract Objectives. Understand How Discontinuous Short Glass Fiber
Fiber reinforcement of a resin modified glass ionomer cement a b b a Carina B. Tanaka , Frances Ershad , Ayman Ellakwa , Jamie J. Kruzic * a - School of Mechanical and Manufacturing Engineering, University of New South Wales (UNSW Sydney), Sydney NSW 2052, Australia b - School of Dentistry, The University of Sydney, Westmead NSW 2145, Australia *Corresponding author. Jamie J. Kruzic Address: School of Mechanical and Manufacturing Engineering, University of New South Wales (UNSW Sydney), Sydney NSW 2052, Australia. Tel.: +61 2 9385 4017 E-mail address: [email protected] Abstract Objectives. Understand how discontinuous short glass fibers and braided long fibers can be effectively used to reinforce a resin modified glass ionomer cement (RMGIC) for carious lesions restorations. Methods. Two control groups (powder/liquid kit and capsule) were prepared from a light cured RMGIC. Either discontinuous short glass fibers or braided polyethylene fiber ribbons were used as a reinforcement both with and without pre-impregnation with resin. For the former case, the matrix was the powder/liquid kit RMGIC, and for the latter case the matrix was the capsule form. Flexural strength was evaluated by three-point beam bending and fracture toughness was evaluated by the single-edge V-notch beam method. Compressive strength tests were performed on cylindrical samples. Results were compared by analysis of variances and Tukey’s post-hoc test. Flexural strength data were analyzed using Weibull statistical analysis. Results. The short fiber reinforced RMGIC both with and without pre-impregnation showed a significant increase of ~50% in the mean flexural strength and 160 – 220% higher fracture toughness compared with the powder/liquid RMGIC control.