All About Fibers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
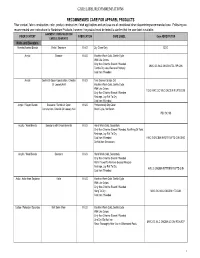
Care Label Recommendations
CARE LABEL RECOMMENDATIONS RECOMMENDED CARE FOR APPAREL PRODUCTS Fiber content, fabric construction, color, product construction, finish applications and end use are all considered when determining recommended care. Following are recommended care instructions for Nordstrom Products, however; the product must be tested to confirm that the care label is suitable. GARMENT/ CONSTRUCTION/ FIBER CONTENT FABRICATION CARE LABEL Care ABREVIATION EMBELLISHMENTS Knits and Sweaters Acetate/Acetate Blends Knits / Sweaters K & S Dry Clean Only DCO Acrylic Sweater K & S Machine Wash Cold, Gentle Cycle With Like Colors Only Non-Chlorine Bleach If Needed MWC GC WLC ONCBIN TDL RP CIIN Tumble Dry Low, Remove Promptly Cool Iron If Needed Acrylic Gentle Or Open Construction, Chenille K & S Turn Garment Inside Out Or Loosely Knit Machine Wash Cold, Gentle Cycle With Like Colors TGIO MWC GC WLC ONCBIN R LFTD CIIN Only Non-Chlorine Bleach If Needed Reshape, Lay Flat To Dry Cool Iron If Needed Acrylic / Rayon Blends Sweaters / Gentle Or Open K & S Professionally Dry Clean Construction, Chenille Or Loosely Knit Short Cycle, No Steam PDC SC NS Acrylic / Wool Blends Sweaters with Embelishments K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed, No Wring Or Twist Reshape, Lay Flat To Dry Cool Iron If Needed HWC S ONCBIN NWOT R LFTD CIIN DNID Do Not Iron Decoration Acrylic / Wool Blends Sweaters K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed Roll In Towel To Remove Excess Moisture Reshape, Lay Flat To Dry HWC S ONCBIN RITTREM -

Choosing the Proper Short Cut Fiber for Your Nonwoven Web
Choosing The Proper Short Cut Fiber for Your Nonwoven Web ABSTRACT You have decided that your web needs a synthetic fiber. There are three important factors that have to be considered: generic type, diameter, and length. In order to make the right choice, it is important to know the chemical and physical characteristics of the numerous man-made fibers, and to understand what is meant by terms such as denier and denier per filament (dpf). PROPERTIES Denier Denier is a property that varies depending on the fiber type. It is defined as the weight in grams of 9,000 meters of fiber. The current standard of denier is 0.05 grams per 450 meters. Yarn is usually made up of numerous filaments. The denier of the yarn divided by its number of filaments is the denier per filament (dpf). Thus, denier per filament is a method of expressing the diameter of a fiber. Obviously, the smaller the denier per filament, the more filaments there are in the yarn. If a fairly closed, tight web is desired, then lower dpf fibers (1.5 or 3.0) are preferred. On the other hand, if high porosity is desired in the web, a larger dpf fiber - perhaps 6.0 or 12.0 - should be chosen. Here are the formulas for converting denier into microns, mils, or decitex: Diameter in microns = 11.89 x (denier / density in grams per milliliter)½ Diameter in mils = diameter in microns x .03937 Decitex = denier x 1.1 The following chart may be helpful. Our stock fibers are listed along with their density and the diameter in denier, micron, mils, and decitex for each: Diameter Generic Type -

Investigation of the Mechanical Properties of a Carbon Fibre-Reinforced Nylon Filament for 3D Printing
machines Article Investigation of the Mechanical Properties of a Carbon Fibre-Reinforced Nylon Filament for 3D Printing Flaviana Calignano 1,* , Massimo Lorusso 2 , Ignanio Roppolo 3 and Paolo Minetola 1 1 Department of Management and Production Engineering, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Turin, Italy; [email protected] 2 Istituto Italiano di Tecnologia, Center for Sustainable Future Technologies IIT@Polito, Corso Trento 21, 10129 Turin, Italy; [email protected] 3 Department of Applied Science and Technology, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Turin, Italy; [email protected] * Correspondence: fl[email protected]; Tel.: +39-011-090-7218 Received: 19 July 2020; Accepted: 2 September 2020; Published: 4 September 2020 Abstract: Additive manufacturing (i.e., 3D printing) has rapidly developed in recent years. In the recent past, many researchers have highlighted the development of in-house filaments for fused filament fabrication (FFF), which can extend the corresponding field of application. Due to the limited mechanical properties and deficient functionality of printed polymer parts, there is a need to develop printable polymer composites that exhibit high performance. This study analyses the actual mechanical characteristics of parts fabricated with a low-cost printer from a carbon fibre-reinforced nylon filament. The results show that the obtained values differ considerably from the values presented in the datasheets of various filament suppliers. Moreover, the hardness and tensile strength are influenced by the building direction, the infill percentage, and the thermal stresses, whereas the resilience is affected only by the building direction. Furthermore, the relationship between the mechanical properties and the filling factor is not linear. -

Long Term Loading and Additional Material Properties of Vectran Fabric for Inflatable Structure Applications
Graduate Theses, Dissertations, and Problem Reports 2010 Long Term Loading and Additional Material Properties of Vectran Fabric for Inflatable Structure Applications Timothy L. Weadon Jr. West Virginia University Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Weadon, Timothy L. Jr., "Long Term Loading and Additional Material Properties of Vectran Fabric for Inflatable Structure Applications" (2010). Graduate Theses, Dissertations, and Problem Reports. 4669. https://researchrepository.wvu.edu/etd/4669 This Thesis is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Thesis in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Thesis has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. Long Term Loading and Additional Material Properties of Vectran Fabric for Inflatable Structure Applications Timothy L Weadon, Jr Thesis submitted to the College of Engineering and Mineral Resources at West Virginia University in partial fulfillment of the requirements -

16 Textiles in Defence* Richard a Scott Defence Clothing and Textiles Agency, Science and Technology Division, Flagstaff Road, Colchester, Essex CO2 7SS, UK
16 Textiles in defence* Richard A Scott Defence Clothing and Textiles Agency, Science and Technology Division, Flagstaff Road, Colchester, Essex CO2 7SS, UK 16.1 Introduction To be prepared for War is one of the most effectual means of preserving Peace (George Washington, 1790)1 Defence forces on land, sea, or air throughout the world are heavily reliant on tech- nical textiles of all types – whether woven, knitted, nonwoven, coated, laminated, or other composite forms. Technical textiles offer invaluable properties for military land forces in particular, who are required to move, live, survive and fight in hostile environments. They have to carry or wear all the necessities for comfort and sur- vival and thus need the most lightweight, compact, durable, and high performance personal clothing and equipment. The life-critical requirements for protecting indi- viduals from both environmental and battlefield threats have ensured that the major nations of the world expend significant resources in developing and providing the most advanced technical textiles for military use. 16.2 Historical background Military textile science is not new, and one of the earliest documented studies can probably be credited to Count Rumford, or Benjamin Thompson. Rumford was an American army colonel and scientist who issued a paper in 1792 entitled ‘Philo- sophical Transactions’, which reported on the importance of internally trapped air in a range of textile fabrics to the thermal insulation provided by those fabrics.2 He was awarded the Copley Medal for his paper, as the significance of his discovery was recognised immediately. * Copyright MOD (1997) DCTA, Colchester, Essex CO2 7SS 426 Handbook of technical textiles 16.2.1 Pre-Twentieth century Up until the end of the 19th century military land battles were fought at close quar- ters by individual engagements. -

Analysis of the Polyester Clothing Value Chain to Identify Key Intervention Points for Sustainability Cristina Palacios‑Mateo* , Yvonne Van Der Meer and Gunnar Seide
Palacios‑Mateo et al. Environ Sci Eur (2021) 33:2 https://doi.org/10.1186/s12302‑020‑00447‑x REVIEW Open Access Analysis of the polyester clothing value chain to identify key intervention points for sustainability Cristina Palacios‑Mateo* , Yvonne van der Meer and Gunnar Seide Abstract Clothing is one of the primary human needs, and the demand is met by the global production of thousands of tons of textile fbers, fabrics and garments every day. Polyester clothing manufactured from oil‑based polyethylene tereph‑ thalate (PET) is the market leader. Conventional PET creates pollution along its entire value chain—during the produc‑ tion, use and end‑of‑life phases—and also contributes to the unsustainable depletion of resources. The consumption of PET garments thus compromises the quality of land, water and air, destroys ecosystems, and endangers human health. In this article, we discuss the diferent stages of the value chain for polyester clothing from the perspective of sustainability, describing current environmental challenges such as pollution from textile factory wastewater, and microfbers released from clothing during the laundry cycle. We also consider potential solutions such as enhanced reuse and recycling. Finally, we propose a series of recommendations that should be applied to polyester clothing at all stages along the value chain, ofering the potential for meaningful and efective change to improve the environ‑ mental sustainability of polyester textiles on a global scale. Keywords: PET, Textiles, Value chain, Environmental sustainability, Microfbers, Pollution, Recycling, Life cycle Introduction with 2019 estimates in Germany suggesting an average Te global volume of fber production for textile manu- lifetime of only 4.4 years [5]. -

Performance Evaluation of 3-D Basalt Fiber Reinforced Concrete & Basalt
Highway IDEA Program Performance Evaluation of 3-D Basalt Fiber Reinforced Concrete & Basalt Rod Reinforced Concrete Final Report for Highway IDEA Project 45 Prepared by: V Ramakrishnan and Neeraj S. Tolmare, South Dakota School of Mines & Technology, Rapid City, SD Vladimir B. Brik, Research & Technology, Inc., Madison, WI November 1998 IDEA PROGRAM F'INAL REPORT Contract No. NCHRP-45 IDEA Program Transportation Resea¡ch Board National Research Council November 1998 PERFORMANCE EVALUATION OF 3.I) BASALT ITIBER REINFORCED CONCRETE & BASALT R:D RETNFORCED CONCRETE V. Rarnalaishnan Neeraj S. Tolmare South Dakota School of Mines & Technolory, Rapid City, SD Principal Investigator: Madimir B. Brik Research & Technology, lnc., Madison, WI INNOVATIONS DESERVING EXPLORATORY ANALYSIS (IDEA) PROGRAMS MANAGED BY THE TRANSPORTATION RESEARCH BOARD (TRB) This NCHRP-IDEA investigation was completed as part of the National Cooperative Highway Research Program (NCHRP). The NCHRP-IDEA program is one of the four IDEA programs managed by the Transportation Research Board (TRB) to foster innovations in highway and intermodal surface transportation systems. The other three IDEA program areas are Transit-IDEA, which focuses on products and results for transit practice, in support of the Transit Cooperative Research Program (TCRP), Safety-IDEA, which focuses on motor carrier safety practice, in support of the Federal Motor Carrier Safety Administration and Federal Railroad Administration, and High Speed Rail-IDEA (HSR), which focuses on products and results for high speed rail practice, in support of the Federal Railroad Administration. The four IDEA program areas are integrated to promote the development and testing of nontraditional and innovative concepts, methods, and technologies for surface transportation systems. -

Ballistic Materials Handbook
Ballistic materials handbook Aramids by Teijin 2 Aramids by Teijin Handbook ballistic materials 3 Teijin Aramid and ballistic protection The intensity of threatening environments for law enforcement, emergency responders and defense forces around the world is becoming higher and the people operating in these hostile environments need to take greater care than ever. This growing threat of violence has led to an increasing demand for ballistic protection. At Teijin Aramid we are dedicated to providing this protection with our high performance para-aramid fiber Twaron® and UHMWPE Endumax® film. With excellent energy absorption Index properties, tenacity and impact resistance, Twaron® and Endumax® offer effective and comfortable ballistic protection Teijin Aramid and ballistic protection 2 solutions with an outstanding cost-performance ratio. In the Twaron® ballistic yarns 4 more than 30 years that Twaron® has been available on the Twaron® ballistic fabrics 7 market, it has helped to save thousands of lives worldwide. Ballistic laminates & coated fabrics 12 Key applications for Twaron® and Endumax® include bullet/ Uni-directional laminates 17 fragment/stab/spike resistant vests, helmets and ballistic Ballistic prepregs 19 protection of vehicles, aircrafts and vessels. Cross sections 21 Endumax® Shield 22 2 Aramids by Teijin Handbook ballistic materials 3 Soft ballistic protection The threats to modern armies and law enforcement forces have multiplied, creating the need for protection from all kinds of bullets and fragments as well as stabbing with sharp objects. And these days it’s not only soldiers and policemen who are facing increased threats; prison guards, cash carriers and private individuals also need to be protected. -

FL.Datasheet Kevlar® Distribution Program.Indd
MOVING HIGH PERFORMANCE FIBERS FORWARD KEVLAR® DISTRIBUTION PROGRAM FIBERS PROCESSES PRODUCTS WHY FIBER-LINE® DUPONTFIBER TM OPTICAL DISTRIBUTION CABLES PROGRAM? Key Features FIBER-LINE® values its relationships with both its customers and • Purchase small quantities of Kevlar® suppliers. Over the past several years, FIBER-LINE® and DuPontTM have Para-Aramid formed a strong partnership based upon the synergies between both • Many deniers & types available organizations. • Customize your Kevlar® solution with FIBER-LINE® performance adding processes FIBER-LINE®’s ability to add value to the already attractive properties of both Kevlar® Para-Aramid & Nomex® Meta-Aramid creates more opportunity in the market place to provide solution driven products to a diverse range of markets. Because FIBER-LINE® already processes so many different types and deniers of both Kevlar® & Nomex®, FIBER-LINE® have been authorized by DuPontTM to distribute small quantities of these fibers to an ever- growing customer base. Through this program, we hope to introduce businesses of all sizes to the benefit of aramid fibers. Contact us today for small order quantity orders. Available Deniers 200, 380, 400, 750AP, 800AP, 1000, 1000AP, 1420, 1500, 1500AP, 1500BK(Black), 2160, 2250, 2840, 3000, 7100. MOVING HIGH PERFORMANCE FIBERS FORWARD KEVLAR® PARA-ARAMID (HM) BARE FIBER PERFORMANCE Chemical Chemical Chemical Abrasion Yarn on Yarn Ultraviolet (UV) Flame Resistance Resistance Resistance Resistance Abrasion Resistance Resistance (Acid) (Alkali) (Organic Solvent) P O X P P P P CHEMICAL COMPATIBILITY Chemical Resistance to Acid: Degrades in Formic, Hydrochloric, and Sodium Hydroxide acid. Chemical Resistance to Alkali: Strong alkalis will attack at high temperature or concentration. Chemical Resistance to Organic Solvent: Degrades moderately in Carbon Tetrachloride and Ethylene Glycol/Water. -

Endumax® – an Ultra-Strong Thin Film with a High Modulus Contents
Endumax® – an ultra-strong thin film with a high modulus Contents What is Endumax? 3 How is Endumax produced? 4 What types of Endumax are available? 6 What can Endumax be used for? 8 Endumax – a unique combination of properties 10 About Teijin Teijin is a technology-driven global group, based in Japan, offering advanced solutions in the areas of sustainable transportation, information and electronics, safety and protection, environment and energy, and healthcare. Its main fields of operation are high-performance fibers (e.g., aramid, carbon fibers and composites), healthcare, films, resin & plastic processing, polyester fibers, product conversion and IT. The group has some 150 companies and around 17,000 employees spread over 20 countries worldwide. Endumax is part of Teijin’s high- performance fibers business, which also produces the aramid fibers Twaron, Technora and Teijinconex. Teijin’s high-performance fibers business is based in Arnhem, The Netherlands. 2 What is Endumax? Endumax film is a new, patented high-performance film developed and made by Teijin. It can be used in a wide variety of products for various market segments – anywhere, in fact, where there is a need for superior strength, safety, light weight or durability. For example, Endumax is used worldwide in applications and markets ranging from ballistic protection (armoring and bulletproof vests), ropes and cables to cargo containers, laminated sails and even loudspeakers. The film shape of Endumax allows for easy processing and seamless integration into the application of the customer. Super-strong and more Weight-for-weight, Endumax is 11 times stronger than steel. But Endumax offers more than incredible strength. -

A Perspective on High-Performance CNT Fibres for Structural Composites
A Perspective on High-performance CNT fibres for Structural Composites Anastasiia Mikhalchan, Juan José Vilatela IMDEA Materials Institute, C/Eric Kandel 2, Getafe, Madrid, Spain, 28906 This review summarizes progress on structural composites with carbon nanotube (CNT) fibres. It starts by analyzing their development towards a macroscopic ensemble of elongated and aligned crystalline domains alongside the evolution of the structure of traditional high- performance fibres. Literature on tensile properties suggests that there are two emerging grades: highly aligned fibres spun from liquid crystalline solutions, with high modulus (160 GPa/SG) and strength (1.6 GPa/SG), and spun from aerogels of ultra-long nanotubes, combining high strength and fracture energy (up to 100J/g). The fabrication of large unidirectional fabrics with similar properties as the fibres is presently a challenge, which CNT alignment remaining a key factor. A promising approach is to produce fabrics directly from aerogel filaments without having to densify and handle individual CNT fibres. Structural composites of CNT fibres have reached longitudinal properties of about 1 GPa strength and 140 GPa modulus, however, on relatively small samples. In general, there is need to demonstrate fabrication of large CNT fibre laminate composites using standard fabrication routes and to study longitudinal and transverse mechanical properties in tension and compression. Complementary areas of development are interlaminar reinforcement Corresponding Author. E-mail: [email protected] (Anastasiia Mikhalchan) Corresponding Author. E-mail: [email protected] (Juan José Vilatela) 1 with CNT fabric interleaves, and multifunctional structural composites with energy storage or harvesting functions. 2 TABLE OF CONTENTS PART 1. State-of-the-art of CNT fibres 5 1.1 Synthesis and hierarchical structure of CNT fibres 5 1.2 Mechanical properties of CNT fibres 8 1.3 Historical retrospective on R&D of commercial high-performance fibres and CNT 11 fibres PART 2. -

Tensile Strength of C/C Composites
16 TH INTERNATIONAL CONFERENCE ON COMPOSITE MATERIALS TENSILE STRENGTH OF C/C COMPOSITES Jun Koyanagi*, Hiroshi Hatta*, Yasuo Kogo**, Ichiro Shiota*** *Institute of Space and Astronautical Science, Japan Aerospace Exploration Agency, **Department of Materials Science and Technology, Tokyo University of Science *** Department of Materials Science and Technology, Kogakuin University Keywords : C/C composites, tensile strength, fracture mechanism ABSTRACT changing heat treatment temperature, and the tensile Fracture mechanism and model based on it in strength and interfacial strength were measured. In Carbon-carbon composites are studied. Interfacial addition, comparing these results with their fracture strength between reinforcing fiber and matrix is a patterns, a tensile fracture model of C/Cs was potential candidate governing the tensile fracture of proposed. C/Cs. However, The mechanisms connecting the tensile strength and the factor have not yet been 2. EXPERIMENTAL PROCEDURE clarified. In the present study, tensile fracture 2.1 Materials patterns of various C/C composites with changing Two types of C/Cs were examined. These the interfacial strength were observed in detail, and C/Cs were different only in their reinforcing fibers, a fundamental tensile fracture model of C/C K321 and K633 by Mitsubishi Sanshi Co. Japan. composites in accordance with the experimental These fibers were fabricated from the same observations were proposed. The analytical result precursor but differed only in HTT. Hereafter, the shows reasonable agreement with the experimental C/Cs reinforced with K321 and K633 will be results, and verifies that the fracture mechanism is referred to as K321-C/C and K633-C/C, respectively. consistent. Both C/Cs were of a symmetric 0°/90° cross-ply lamination, fabricated from carbon fiber reinforced phenolic resin matrix composites, CFRPs, with a 1.