Macor®- Glass Ceramics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ocean Drilling Program Initial Reports Volume
Sigurdsson, H., Leckie, R.M., Acton, G.D., et al., 1997 Proceedings of the Ocean Drilling Program, Initial Reports, Vol. 165 2. EXPLANATORY NOTES1 Shipboard Scientific Party2 INTRODUCTION Shipboard Scientific Procedures Numbering of Sites, Holes, Cores, and Samples In this chapter, we have assembled information that documents our scientific methods. This information concerns only shipboard Drilling sites are numbered consecutively from the first site methods described in the site reports in the Initial Reports volume of drilled by the Glomar Challenger in 1968. A site number refers to the Leg 165 Proceedings of the Ocean Drilling Program (ODP). one or more holes drilled while the ship was positioned over one Methods for shore-based analyses of Leg 165 data will be described acoustic beacon. Multiple holes may be drilled at a single site by pull- in the individual scientific contributions to be published in the Scien- ing the drill pipe above the seafloor (out of the hole), moving the ship tific Results volume. some distance from the previous hole, and then drilling another hole. Coring techniques and core handling, including the numbering of In some cases, the ship may return to a previously occupied site to sites, holes, cores, sections, and samples were the same as those re- drill additional holes. ported in previous Initial Reports volumes of the Proceedings of the For all ODP drill sites, a letter suffix distinguishes each hole Ocean Drilling Program with two exceptions: The core sections drilled at the same site. For example, the first hole drilled is assigned from Holes 1002D and 1002E in the Cariaco Basin were not split dur- the site number modified by the suffix "A," the second hole takes the ing Leg 165; instead, they were transported to the Gulf Coast Repos- site number and suffix "B," and so forth. -

Tevilas Keilim
STAR-K Kosher Classroom HALACHOS OF TEVIFOOD UTENSIL TEVILA GUIDELINELASFOOD UTENSIL TEVILAKEILIM GUIDELINE FOOD UTENSIL TEVILA GUIDELINE Earthenware, Non-Glazed Porcelain Enamel Tevila w/o Brocha Aluminum Pans, Disposable Tevila with Brocha No Tevila to be used more than once Dull Finish, e.g. Flower Pot Racks, Cooling Tevila w/o Brocha George Foreman Grill Tevila w/o Brocha Aluminum Pans, Disposable Tevila w/o Brocha Racks, Oven No Tevila to be used only once (including Pyrex, Duralex & Corelle) Glass Tevila with Brocha Rolling Pins Metal or Wood No Tevila Blech No Tevila Hot Air Popcorn Maker, Metal Tevila with Brocha Sandwich Maker Tevila w/o Brocha Blender -Handheld Immersion Hot Water Urn, Metal Tevila with Brocha Wand with metal blade on bottom Tevila with Brocha Sink Racks, Stainless Steel No Tevila Ice Cream Scooper Tevila with Brocha Bone No Tevila Spatula, Metal Tevila with Brocha Knife, Arts & Crafts No Tevila Brush, Pastry No Tevila Stoneware Tevila w/o Brocha Knife Sharpener No Tevila Brush for Grill, Metal No Tevila Stoneware, Non-Glazed No Tevila Meat Thermometer No Tevila Can Opener No Tevila Storage Utensils, Glass Meat Tenderizer Hammer, not brought to the table No Tevila Cans, Reusable Empty Metal No Tevila if opened by a Yehudi No Tevila Metal Cutlery Tevila with Brocha Styrofoam No Tevila China, Bone Tevila w/o Brocha Tea Kettle, Corelle Tevila with Brocha Metal Flour & Sugar Storage Tevila w/o Brocha China, Glazed Tevila w/o Brocha Canisters Thermos (Glass Insert) Tevila with Brocha Colander, Metal Tevila with Brocha -
Catalog Holding List A-E in PDF Form
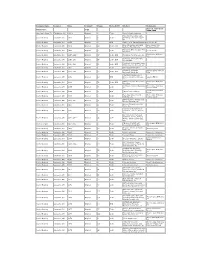
Company Name Location Date Original? Pages Color-B/W? Product Comments See U.S. Glass Circa Adams & Co. Copy 1890-1900 Akro Agate Glass Co. Clarksburg, WV 1940’s Original 4 Color Akroite floral containers Decorated beverage ware, Anchor Hocking Lancaster, OH 1942 Original 11 Color Fire-King oven ware & jade- ite Anchor Hocking Lancaster, OH 1953 Original 4 B/W Cape Cod or Sandwich pattern Loose sheets Decorated glass, Sandwich Some pages have Anchor Hocking Lancaster, OH 1954 Original 44 Color, B/W pattern, Fire-King, etc. pictures cut out Turquoise Blue & Copper Tint Anchor Hocking Lancaster, OH 1956 Original 5 Color Loose sheets Fire-King Plus 4 page B/W price Anchor Hocking Lancaster, OH 1957-1958 Original 47 Color, B/W Fire-King, Forest Green, etc. list Fire-King, beverage items, Anchor Hocking Lancaster, OH 1959-1960 Original 56 Color, B/W kitchenware Fire-King, kitchenware, some Anchor Hocking Lancaster, OH 1960-1961 Original 59 Color, B/W Early American Pres-cut, etc. Anchor Hocking Lancaster, OH 1961 Original 1 Color Desert gold stemware Fire-King, Sandwich, Several pages with cut Anchor Hocking Lancaster, OH 1961-1962 Original 67 Color, B/W beverage items, etc. outs “Institutional Glassware”, Anchor Hocking Lancaster, OH 1963 Original 12 B/W Stems, tumblers, Ashtrays, Catalog HR 63 etc. Mostly beverage ware, some Plus 8 page B/W price Anchor Hocking Lancaster, OH 1964 Original 78 Color, B/W Fire-King list Tumblers, Prescut, Ovenware, Plus 2 page B/W price Anchor Hocking Lancaster, OH 1965 Original 78 Color etc. revision sheet Loose sheet, includes Anchor Hocking Lancaster, OH 1965 Original 2 B/W “Bravo” Cut Tumblers prices Beverage Ware, Prescut, , Plus 1 page B/W price Anchor Hocking Lancaster, OH 1966 Original 80 Color Jade-ite, etc. -

Glossary of Terms Abrisa Technologies Your Single Source Optics Partner!
Glossary of Terms Abrisa Technologies Your Single Source Optics Partner! December 2015 200 South Hallock Drive, Santa Paula, CA 93060 • (877) 622-7472 • FAX (805) 525-8604 • www.abrisatechnologies.com Glossary of Terms - 12/15 2 of 13 Acid Etching This process for the decoration of glass involves the application of hydrofluoric acid to the glass surface. Hydrofluoric acid vapors or baths of hydrofluoric acid salts may be used to give glass a matte, frosted appearance (similar to that obtained by surface sandblasting), as found in lighting glass. Glass designs can be produced by coating the glass with wax and then inscribing the desired pattern through the wax layer. When applied, the acid will corrode the glass but not attack the wax-covered areas. Alumina-silicate Glass Alumina (aluminum oxide Al2O3) is added to the glass batch in the form of commonly found feldspars containing alkalis in order to help improve chemical resistance and mechanical strength, and to increase viscosity at lower temperatures. Angle of Incidence The angle formed between a ray of light striking a surface and the normal line (the line perpendicular to the surface at that point). Annealing Under natural conditions, the surface of molten glass will cool more rapidly than the center. This results in internal stress- es which may cause the glass sheet or object to crack, shatter or even explode some time later. The annealing process is designed to eliminate or limit such stresses by submitting the glass to strictly controlled cooling in a special oven known as a “lehr”. Inside the lehr, the glass is allowed to cool to a temperature known as the “annealing point”. -

The Pennsylvania State University
The Pennsylvania State University The Graduate School ADVENTURES IN HIGH DIMENSIONS: UNDERSTANDING GLASS FOR THE 21ST CENTURY A Dissertation in Material Science and Engineering by Collin James Wilkinson © 2021 Collin James Wilkinson Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy May 2021 ii The dissertation of Collin Wilkinson was reviewed and approved by the following: John Mauro Professor of Materials Science and Engineering Chair, Intercollege Graduate Degree Program Associate Head for Graduate Education, Materials Science and Engineering Dissertation Advisor Chair of Committee Seong Kim Professor of Chemical Engineering Professor of Materials Science and Engineering Ismaila Dabo Associate Professor of Materials Science and Engineering Susan Sinnott Professor of Materials Science and Engineering Professor of Chemistry Head of the Department of Materials Science and Engineering iii Abstract Glass is infinitely variable. This complexity stands as a promising technology for the 21st century since the need for environmentally friendly materials has reached a critical point due to climate change. However, such a wide range of variability makes new glass compositions difficult to design. The difficulty is only exaggerated when considering that not only is there an infinite variability in the compositional space, but also an infinite variability thermal history of a glass and in the crystallinity of glass-cearmics. This means that even for a simple binary glass there are at least 3 dimensions that have to be optimized. To resolve this difficulty, it is shown that energy landscapes can capture all three sets of complexity (composition, thermal history, and crystallinity). The explicit energy landscape optimization, however, has a large computational cost. -

Mead Art Museum Andrew W. Mellon Faculty Seminar: Jan 15 and 16, 2015
Mead Art Museum Andrew W. Mellon Faculty Seminar: Jan 15 and 16, 2015 Looking at Glass through an Interdisciplinary Lens: Teaching and Learning with the Mead’s Collection Books: Bach, Hans and Norbert Neuroth, eds. The Properties of Optical Glass. Berlin: Springer-Verlag, 1995. Barr, Sheldon. Venetian Glass: Confections in Glass, 1855-1914. New York: Harry N. Abrams, 1998. Battie, David and Simon Cottle, eds. Sotheby's Concise Encyclopedia of Glass. London: Conran Octopus, 1991. Blaszczyk, Regina Lee. Imagining Consumers, Design and Innovation from Wedgwood to Corning. Baltimore: Johns Hopkins University Press, 2000. Bradbury, S. The Evolution of the Microscope. Oxford: Pergamon Press, 1967. Busch, Jason T., and Catherine L. Futter. Inventing the Modern World: Decorative Arts at the World’s Fairs, 1951-1939. New York, NY: Skira Rizzoli, 2012. Carboni, Stefano and Whitehouse, David. Glass of the Sultans. New York: Metropolitan Museum of Art; Corning, NY: The Corning Museum of Glass; Athens: Benaki Museum; New Haven and London: Yale University Press, 2001. Charleston, Robert J. Masterpieces of glass: a world history from the Corning Museum of Glass. 2nd ed.: New York, Harry N. Abrams, 1990. The Corning Museum of Glass. Innovations in Glass. Corning, New York: The Corning Museum of Glass, 1999. Lois Sherr Dubin. The History of Beads: from 30,000 B.C. to the present. London: Thames & Hudson, 2006. Fleming, Stuart. Roman Glass: Reflections of Everyday Life. Philadelphia: University of Pennsylvania Museum, 1997. ----Roman Glass: Reflections on Cultural Change. Philadelphia: University of Pennsylvania Museum of Archaeology and Anthropology, 1999. 1 Frelinghuysen, Alice Cooney. Louis Comfort Tiffany at the Metropolitan Museum. -

Lecture #16 Glass-Ceramics: Nature, Properties and Processing Edgar Dutra Zanotto Federal University of São Carlos, Brazil [email protected] Spring 2015
Glass Processing Lecture #16 Glass-ceramics: Nature, properties and processing Edgar Dutra Zanotto Federal University of São Carlos, Brazil [email protected] Spring 2015 Lectures available at: www.lehigh.edu/imi Sponsored by US National Science Foundation (DMR-0844014) 1 Glass-ceramics: nature, applications and processing (2.5 h) 1- High temperature reactions, melting, homogeneization and fining 2- Glass forming: previous lectures 3- Glass-ceramics: definition & applications (March 19) Today, March 24: 4- Composition and properties - examples 5- Thermal treatments – Sintering (of glass powder compactd) or -Controlled nucleation and growth in the glass bulk 6- Micro and nano structure development April 16 7- Sophisticated processing techniques 8- GC types and applications 9- Concluding remmarks 2 Review of Lecture 15 Glass-ceramics -Definition -History -Nature, main characteristics -Statistics on papers / patents - Properties, thermal treatments micro/ nanostructure design 3 Reading assignments E. D. Zanotto – Am. Ceram. Soc. Bull., October 2010 Zanotto 4 The discovery of GC Natural glass-ceramics, such as some types of obsidian “always” existed. René F. Réaumur – 1739 “porcelain” experiments… In 1953, Stanley D. Stookey, then a young researcher at Corning Glass Works, USA, made a serendipitous discovery ...… 5 <rms> 1nm Zanotto 6 Transparent GC for domestic uses Zanotto 7 Company Products Crystal type Applications Photosensitive and etched patterned Foturan® Lithium-silicate materials SCHOTT, Zerodur® β-quartz ss Telescope mirrors Germany -

New Glass Review 10.Pdf
'New Glass Review 10J iGl eview 10 . The Corning Museum of Glass NewG lass Review 10 The Corning Museum of Glass Corning, New York 1989 Objects reproduced in this annual review Objekte, die in dieser jahrlich erscheinenden were chosen with the understanding Zeitschrift veroffentlicht werden, wurden unter that they were designed and made within der Voraussetzung ausgewahlt, dal3 sie the 1988 calendar year. innerhalb des Kalenderjahres 1988 entworfen und gefertigt wurden. For additional copies of New Glass Review, Zusatzliche Exemplare des New Glass Review please contact: konnen angefordert werden bei: The Corning Museum of Glass Sales Department One Museum Way Corning, New York 14830-2253 (607) 937-5371 All rights reserved, 1989 Alle Rechtevorbehalten, 1989 The Corning Museum of Glass The Corning Museum of Glass Corning, New York 14830-2253 Corning, New York 14830-2253 Printed in Dusseldorf FRG Gedruckt in Dusseldorf, Bundesrepublik Deutschland Standard Book Number 0-87290-119-X ISSN: 0275-469X Library of Congress Catalog Card Number Aufgefuhrt im Katalog der KongreB-Bucherei 81-641214 unter der Nummer 81-641214 Table of Contents/lnhalt Page/Seite Jury Statements/Statements der Jury 4 Artists and Objects/Kunstler und Objekte 10 Bibliography/Bibliographie 30 A Selective Index of Proper Names and Places/ Verzeichnis der Eigennamen und Orte 53 er Wunsch zu verallgemeinern scheint fast ebenso stark ausgepragt Jury Statements Dzu sein wie der Wunsch sich fortzupflanzen. Jeder mochte wissen, welchen Weg zeitgenossisches Glas geht, wie es in der Kunstwelt bewer- tet wird und welche Stile, Techniken und Lander maBgeblich oder im Ruckgang begriffen sind. Jedesmal, wenn ich mich hinsetze und einen Jurybericht fur New Glass Review schreibe (dies ist mein 13.), winden he desire to generalize must be almost as strong as the desire to und krummen sich meine Gedanken, um aus den tausend und mehr Dias, Tprocreate. -

Vitrification of Historic and Future High Level Nuclear Wastes Within Alkali Borosilicate Glasses
Vitrification of historic and future high level nuclear wastes within alkali borosilicate glasses Andrew James Connelly M.Eng. A Thesis submitted to the Department of Engineering Materials at the University of Sheffield in partial fulfilment of the requirement for the Degree of Doctor of Philosophy. February 2008 The University Of Sheffield. Abstract The disposal of highly radioactive and toxic wastes generated by the nuclear industry is one of the biggest challenges facing the world today. Currently, in the UK there is a large legacy waste holding which has been accumulating since nuclear energy was first harnessed during World War 2. Processing of this waste with a view to final disposal is a complex and difficult task. This work investigates one aspect of that process, namely turning this waste into glass (or vitrification). This work uses multiple techniques including x-ray absorption spectroscopy, magic angle spinning nuclear magnetic resonance and molecular dynamic simulations, to investigate the structural role of Zr02 and U 03 within the alkali borosilicate glass used in the UK for waste immobilisation. The effect of these additions on the bulk glass structure and selected glass properties are also explored. In waste glasses Zr occurs as a 6 co-ordinated Zr ion surrounded by Si, B, Na and Li. The effect of Zr02 additions on the bulk glass structure and properties is highly complex. The addition of Zr02 appears to be characterised by a non-linearity in the trends of certain physical and structural parameters. At low levels of Zr02 the level of leaching from the glasses and the co ordination of B increase. -

Technical Glasses
Technical Glasses Physical and Technical Properties 2 SCHOTT is an international technology group with 130 years of ex perience in the areas of specialty glasses and materials and advanced technologies. With our highquality products and intelligent solutions, we contribute to our customers’ success and make SCHOTT part of everyone’s life. For 130 years, SCHOTT has been shaping the future of glass technol ogy. The Otto Schott Research Center in Mainz is one of the world’s leading glass research institutions. With our development center in Duryea, Pennsylvania (USA), and technical support centers in Asia, North America and Europe, we are present in close proximity to our customers around the globe. 3 Foreword Apart from its application in optics, glass as a technical ma SCHOTT Technical Glasses offers pertinent information in terial has exerted a formative influence on the development concise form. It contains general information for the deter of important technological fields such as chemistry, pharma mination and evaluation of important glass properties and ceutics, automotive, optics, optoelectronics and information also informs about specific chemical and physical character technology. Traditional areas of technical application for istics and possible applications of the commercial technical glass, such as laboratory apparatuses, flat panel displays and glasses produced by SCHOTT. With this brochure, we hope light sources with their various requirements on chemical to assist scientists, engineers, and designers in making the physical properties, have led to the development of a great appropriate choice and make optimum use of SCHOTT variety of special glass types. Through new fields of appli products. cation, particularly in optoelectronics, this variety of glass types and their modes of application have been continually Users should keep in mind that the curves or sets of curves enhanced, and new forming processes have been devel shown in the diagrams are not based on precision measure oped. -

Borosilicate Glass Wafers Are an Inexpensive Alternative
Basics of Microstructuring 01 Chapter MicroChemicals® – Fundamentals of Microstructuring www.microchemicals.com/downloads/application_notes.html PRODUCTION AND SPECIFICATIONS OF GLASS WAFERS For applications where neither the high dielectric strength of quartz nor the high transparency is in the ultravi- olet, visible or infrared spectral range or the thermal stability of quartz or quartz glass is required, borosilicate glass wafers are an inexpensive alternative. Borosilicate Glass and Ordinary Glass in Comparison Composition Borosilicate glass consists of approximately 80% of silicon dioxide (SiO2) and approximately 5-15% boron trioxide (B2O3). Other additives are alkaline oxide (Na2O, K2O), aluminium oxide (Al2O3) and alkaline po- tassium oxide (CaO, MgO). Borosilicate glasses typically have a very low iron content, which causes the typical green colour of window glass in order to increase the transparency. Properties Due to its boron content, borosilicate glass exhibit a higher chemical stability against water, many chemi- cals and pharmaceutical products compared to window glass. In addition, borosilicate glass exhibits signifi cantly higher thermal stability against temperature fl uctua- tions due to its thermal expansion coeffi cients, which are less than half as large as compared to window glass. Production of Glass Wafers For the further processing into glass wafers, specifi c borosilicate glass is produced using the “fl oat pro- cess” (Fig. 37). Hereby the molten glass on melted tin forms a fl oating both-side smooth ribbon with a homogeneous thickness. On its way on the tin bath, the temperature is gradually reduced from 1100 down to 600°C until the sheet can be lifted onto rollers. Glass pane Glass melt Tin melt Fig. -

Stability of Materials for Use in Space-Based Interferometric Missions
STABILITY OF MATERIALS FOR USE IN SPACE-BASED INTERFEROMETRIC MISSIONS By ALIX PRESTON A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2010 1 °c 2010 Alix Preston 2 This is dedicated to all who were told they would fail, only to prove them wrong 3 ACKNOWLEDGMENTS Much of this work would not have been made possible if it were not for the help of many graduate and undergraduate students, faculty, and sta®. I would like to thank Ira Thorpe, Rachel Cruz, Vinzenz Vand, and Josep Sanjuan for their help and thoughtful discussions that were instrumental in understanding the nuances of my research. I would also like to thank Gabriel Boothe, Aaron Spector, Benjamin Balaban, Darsa Donelon, Kendall Ackley, and Scott Rager for their dedication and persistence to getting the job done. A special thanks is due for the physics machine shop, especially Marc Link and Bill Malphurs, who spent many hours on the countless projects I needed. Lastly, I would like to thank my advisor, Dr. Guido Mueller, who put up with me, guided me, and supported me in my research. 4 TABLE OF CONTENTS page ACKNOWLEDGMENTS ................................. 4 LIST OF TABLES ..................................... 9 LIST OF FIGURES .................................... 10 KEY TO ABBREVIATIONS ............................... 17 KEY TO SYMBOLS .................................... 19 ABSTRACT ........................................ 20 CHAPTER 1 INTRODUCTION .................................. 22 1.1 Space-Based Missions .............................. 23 1.2 GRACE ..................................... 23 1.3 GRACE Follow-On ............................... 25 1.4 LISA ....................................... 26 1.4.1 Introduction ............................... 26 1.4.2 Sources .................................. 27 1.4.2.1 Cosmological background sources .............