Process for the Purification of Lithium Carbonate Verfahren Zur Reinigung Von Lithiumcarbonat Procede De Purification Du Carbonate De Lithium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 7.449,161 B2 Boryta Et Al
USOO74491.61B2 (12) United States Patent (10) Patent No.: US 7.449,161 B2 Boryta et al. (45) Date of Patent: Nov. 11, 2008 (54) PRODUCTION OF LITHIUM COMPOUNDS 4,207,297 A * 6/1980 Brown et al. ............. 423,179.5 DIRECTLY FROM LITHIUM CONTAINING 4,243,641 A * 1/1981 Ishimori et al. .......... 423/179.5 BRINES 4.261,960 A * 4/1981 Boryta ............. ... 423,179.5 4,271,131. A * 6/1981 Brown et al. ...... ... 423,179.5 4,274,834. A 6/1981 Brown et al. .............. 23,302 R (75) Inventors: Daniel Alfred Boryta, Cherryville, NC 4,463,209 A * 7/1984 Kursewicz et al. .......... 585/467 (US); Teresita Frianeza Kullberg, 4,465,659 A * 8/1984 Cambridge et al. ......... 423,495 Gastonia, NC (US); Anthony Michael 4,588,565 A * 5/1986 Schultze et al. .......... 423,179.5 Thurston, Edmond, OK (US) 4,747.917 A * 5/1988 Reynolds et al. ............ 205,512 4,859,343 A * 8/1989 Frianeza-Kullberg (73) Assignee: Chemetall Foote Corporation, Kings et al. .......................... 210,679 Mountain, NC (US) 4,980,136 A * 12/1990 Brown et al. ... 423,179.5 5,049,233 A 9, 1991 Davis .......................... 216.93 (*) Notice: Subject to any disclaimer, the term of this 5,219,550 A * 6/1993 Brown et al. ............. 423 (419.1 patent is extended or adjusted under 35 5,599,516 A * 2/1997 Bauman et al. .......... 423/179.5 U.S.C. 154(b) by 0 days. 5,939,038 A * 8/1999 Wilkomirsky ............... 423,276 5.993,759 A * 1 1/1999 Wilkomirsky ........... -

Pfs Update Confirms Potential of Low-Cost Lithium Hydroxide Production
For immediate release 17 June 2019 EUROPEAN METALS HOLDINGS LIMITED PFS UPDATE CONFIRMS POTENTIAL OF LOW-COST LITHIUM HYDROXIDE PRODUCTION European Metals Holdings Limited (“European Metals” or “the Company”) is pleased to announce the results from the successful update of the process flowsheet previously developed to enable the production of lithium hydroxide (LiOH.H2O). This work has been completed in conjunction with test-work confirming the production of battery grade lithium hydroxide from Cinovec ore. These results significantly enhance the forecast economics of the Cinovec Project. HIGHLIGHTS (all $ figures in this release are US Dollars and increases refer to the 2017 PFS Lithium Carbonate study): • Net estimated overall cost of production post credits: $3,435 / tonne LiOH.H2O • Project Net Present Value (“NPV”) increases 105% to: $1.108B (post tax, 8%) • Internal Rate of Return (“IRR”) increased 37% to 28.8% (post tax) • Total Capital Cost: $482.6M • Annual production of Battery Grade Lithium Hydroxide: 25,267 tonnes • Studies are based on only 9.3% of reported Indicated Mineral Resource and a mine life of 21 years processing an average of 1.68 Mtpa ore • The process used to produce lithium hydroxide allows for the staging of lithium carbonate and then lithium hydroxide production to minimize capital and startup risk and enables the production of either battery grade lithium hydroxide or carbonate as markets demand European Metals Managing Director Keith Coughlan said, “I am very pleased to report to shareholders on the completion of this update to our 2017 Preliminary Feasibility Study for the Cinovec project which adds significantly to the already robust forecast economics for the project. -

Sonora Project)
SONORA LITHIUM PROJECT FS TECHNICAL REPORT TECHNICAL REPORT ON THE FEASIBILITY STUDY FOR THE SONORA LITHIUM PROJECT, MEXICO January 2018 January 2018 Prepared For Bacanora Minerals Ltd Prepared by Ausenco Services Pty Ltd 144 Montague Rd South Brisbane Australia Effective Date: December 12, 2017 Issue Date: January 25, 2018 101304-FS-0004-Tech Report rev 0.docx SONORA LITHIUM PROJECT FS TECHNICAL REPORT CERTIFICATE OF QUALIFIED PERSON I, Joel A. Carrasco, P.E., do hereby certify thhat: 1. I am a Principal Engineer, Solum Consulting Group, 350 S Jackson st. #454 Denver, Colorado 80209 USA. 2. This certificate applies to the technical report titled “Technical Report on the Feasibility Study for the Sonora Lithium Project, Mexico, January 2018” (the “Technical Report”), prepared for Bacanora Minerals Limited; 3. The Effective Date of the Technical Report is 12 December 2017. 4. I am a graduate of Texas Tech Univerrsity, Texas with a Bachelor of Scieence degree in Civil Engineering. I have worked as a Civil Engineer continuously for a total off 15 years since my graduation from University. My relevant experience has been working as Project Manager for multi-national engineering companies on feasibility studies and engineering designs of tailings facilities; 5. I am registered as a Professional Engineer in the State of Arizona (Licence # 52000). 6. I havve read the definition of “Qualified Person” set out in National Instrument 43-101 (NI 43-101) and certify that by reason of my education, affiliation with a professional association (as defined in NI 43-101) and past relevant work experrience, I fulfil the requirements to bee a “Qualified Person” for the purposes of NI 43-101. -

NI 43-101 Technical Report Preliminary Economic Assessment of the Whabouchi Lithium Deposit and Hydromet Plant
NI 43-101 Technical Report Preliminary Economic Assessment of the Whabouchi Lithium Deposit and Hydromet Plant Prepared for Nemaska Lithium Inc. Prepared by André Laferrière, P. Geo. SGS Canada Inc. Yves Dessureault, Eng. Patrice Live, Eng. BBA Inc. Nicolas Skiadas, Eng. Noël Journeaux, P. Geo., Eng. Journeaux Assoc. Gary H.K. Pearse, M.Sc., P. Eng. Equapolar Consultants Limited Ann Lamontagne, Eng. Lamont Inc. Isabelle Larouche, Eng. Alain Michaud, Eng. Michel Bilodeau, Eng. Céline Charbonneau, Eng. M. Sc. Effective Date: October 2, 2012 Issue Date: November 16, 2012 Revised Date: February 27, 2013 Equapolar Consultants Limited Project Number: 2012-014 Nemaska Lithium Inc. NI 43-101 Technical Report Preliminary Economic Assessment Prepared for Nemaska Lithium Inc. 450, Gare-du-Palais Street, 1st floor Quebec (Quebec) G1K 3X2 Canada Prepared by: Met-Chem Canada Inc. 555, boul. René-Lévesque Ouest, 3e étage Montréal (Québec) H2Z 1B1 February 2013 QPF-009-12/B P:\2012-014\Texte\Rapports\Ni 43-101\Main Report\2012-014 Nemaska NI 43-101 Rev FINAL1.doc Nemaska Lithium Inc. NI 43-101 Technical Report Preliminary Economic Assessment IMPORTANT NOTICE This Report was prepared as a National Instrument 43-101 Technical Report for Nemaska Lithium Inc. (“Nemaska”) by Met-Chem Canada Inc. (“Met-Chem”). The quality of information, conclusions and estimates contained herein is consistent with the level of effort involved in Met-Chem’s services, based on: i) information available at the time of preparation, ii) data supplied by outside sources, and iii) the assumptions, conditions, and qualifications set forth in this Report. This Report is intended for use by Nemaska subject to the terms and conditions of its contract with Met-Chem. -

HYSYS OLI Interface
HYSYS® 2004.2 OLI Interface Reference Guide Copyright October 2005 Copyright © 1981-2005 by Aspen Technology, Inc. All rights reserved. Aspen Accounting.21™, Aspen ACM Model Export, Aspen ACOL™, Aspen ACX™ Upgrade to ACOL™, Aspen Adsim®, Aspen Advisor™, Aspen Aerotran®, Aspen Alarm & Event™, Aspen APLE™, Aspen Apollo™, Aspen AtOMS™, Aspen Batch and Event Extractor, Aspen Batch Plus®, Aspen Batch.21™, Aspen Batch.21™ CBT, Aspen BatchCAD™, Aspen BatchSep™, Aspen Blend Model Library™, Aspen Blend™, Aspen BP Crude Oil Database, Aspen Calc CBT, Aspen Calc™, Aspen Capable-to-Promise®, Aspen CatRef®, Aspen Chromatography®, Aspen Cim-IO Core™, Aspen Cim-IO™ for @AGlance, Aspen Cim-IO™ for ABB 1180/ 1190 via DIU, Aspen Cim-IO™ for Bailey SemAPI, Aspen Cim-IO™ for DDE, Aspen Cim-IO™ for Eurotherm Gauge via DCP, Aspen Cim-IO™ for Fisher-Rosemount Chip, Aspen Cim-IO™ for Fisher-Rosemount RNI, Aspen Cim-IO™ for Foxboro FOXAPI, Aspen Cim-IO™ for G2, Aspen Cim-IO™ for GE FANUC via HCT, Aspen Cim-IO™ for Hitachi Ex Series, Aspen Cim-IO™ for Honeywell TDC 3000 via HTL/access, Aspen Cim-IO™ for Intellution Fix, Aspen Cim-IO™ for Measurex MCN, Aspen Cim-IO™ for Measurex ODX, Aspen Cim-IO™ for Moore Apacs via Nim (RNI), Aspen Cim-IO™ for OPC, Aspen Cim-IO™ for PI, Aspen Cim- IO™ for RSLinx, Aspen Cim-IO™ for SetCim/InfoPlus-X/InfoPlus.21, Aspen Cim-IO™ for Toshiba Tosdic, Aspen Cim-IO™ for ULMA 3D, Aspen Cim-IO™ for Westinghouse, Aspen Cim-IO™ for WonderWare InTouch, Aspen Cim-IO™ for Yokogawa ACG10S, Aspen Cim-IO™ for Yokogawa EW3, Aspen Collaborative Forecasting™, -

WO 2018/157203 Al 07 September 2018 (07.09.2018) WIPO I PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization lllllllllllllllllllllllllllllllllllllllllll^ International Bureau (10) International Publication Number (43) International Publication Date WO 2018/157203 Al 07 September 2018 (07.09.2018) WIPO I PCT (51) International Patent Classification: (74) Agent: SHELSTON IP PTY LTD; Level 21, 60 Margaret C01D 15/08 (2006.01) C22B 3/00 (2006.01) Street, Sydney, New South Wales 2000 (AU). C22B 26/12 (2006.01) C22B 1/00 (2006.01) (81) Designated States (unless otherwise indicated, for every (21) International Application Number: kind of national protection available): AE, AG, AL, AM, PCT/AU2018/050178 AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, (22) International Filing Date: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, 28 February 2018 (28.02.2018) HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, (25) Filing Language: English KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (26) Publication Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (30) Priority Data: SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 2017900694 01 March 2017 (01.03.2017) AU TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (71) Applicant: THE UNIVERSITY OF SYDNEY [AU/AU]; (84) Designated States (unless otherwise indicated, for every Parramatta Road, Sydney, New South Wales 2006 (AU). -

Processes for Preparing Lithium Carbonate Verfahren Zur Herstellung Von Lithiumcarbonat Procédés De Préparation De Carbonate De Lithium
(19) TZZ ¥_T (11) EP 2 855 735 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C25B 1/16 (2006.01) C01D 15/08 (2006.01) 07.03.2018 Bulletin 2018/10 C22B 3/04 (2006.01) C22B 26/12 (2006.01) (21) Application number: 13796576.0 (86) International application number: PCT/CA2013/000526 (22) Date of filing: 30.05.2013 (87) International publication number: WO 2013/177680 (05.12.2013 Gazette 2013/49) (54) PROCESSES FOR PREPARING LITHIUM CARBONATE VERFAHREN ZUR HERSTELLUNG VON LITHIUMCARBONAT PROCÉDÉS DE PRÉPARATION DE CARBONATE DE LITHIUM (84) Designated Contracting States: • PEARSE, Gary AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Ottawa, Ontario K1M 1A3 (CA) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • MACKIE, Stephen Charles PL PT RO RS SE SI SK SM TR Peterborough, Ontario K9L 1N3 (CA) Designated Extension States: • GLADKOVAS, Mykolas BA ME Toronto, Ontario M9P 2A7 (CA) (30) Priority: 30.05.2012 US 201261653035 P (74) Representative: Regimbeau 21.02.2013 US 201361767328 P 20, rue de Chazelles 75847 Paris Cedex 17 (FR) (43) Date of publication of application: 08.04.2015 Bulletin 2015/15 (56) References cited: WO-A1-2011/133165 CA-A1- 2 820 112 (73) Proprietor: Nemaska Lithium Inc. GB-A- 845 511 US-A1- 2011 123 427 Québec, Québec G1K 3X2 (CA) US-A1- 2011 200 508 (72) Inventors: • BOURASSA, Guy Québec, Québec G1C 7G2 (CA) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

THE PRECISE and ACCURATE DETERMINATION of LITHIUM 1IYDROXIDE SOLUTIONS by J
OF LITHIUM HYDROXIDE SOLUTIONS «Vía!, h!i;l Γι: ¡Γ jä -i-f" Bi PBBrø Β 'iîti*7i..f '»«ι!* íSl-'Λ«,*11 «.i>Ib>i 'rtt ΓΙΑΠ*41Α wufuMiMl. · til ' j**r4ÎSiTA.y\ii L». I BUFI Nuclear Research Center Establishment - Belgium Central Bureau for Nuclear Measurements - CBNM tl« ■■# wem mv ;iS* «tå NOTICE ?3mm ' Γ iii Ιίΐ» ''itCiOilllMrj TU i T. *il!líft2T*»liit ' ™ Jit Tuk ii fl-13ίΙ ì 11 This document was prepared under trie sponsorship of the Commission or the European Communities. Neither the Commission of the European Communities, its contractors nor any person acting on their hehalf : ■ l· Α A it Λ i*P¡ maice any warranty or representation, express or implied, with respect to the accuracy, completeness, or usefulness of the information contained in this document, or that the use of any information, apparatus, method, or process disclosed in this document may not infringe privately owned rights; Assume any liability with respect to the use of, or for damages resu from the use of any information apparatus, method or process disc in this document. J* at the price of FF 4.- FB 40, DM 3.20 Lit. 500 When ordering, please quote the EUR number and the title, which are indicated on the cover of each report EUR 3656 e THE PRECISE AND ACCURATE DETERMINATION OF LITHIUM 1IYDROXIDE SOLUTIONS by J. PAUWELS, Y. LE DUIGOU and K.P. LAUER European Atomic Energy Community - EURATOM Joint Nuclear Research Center - Geel Establishment (Belgium) Central Bureau for Nuclear Measurements - CBNM Brussels, December 1967 - 20 Pages - 2 Figures - FB 40 Four methods for the precise determination of lithium hydroxide solutions have been compared : the acid-base titration with hydrochloric acid, tøe acid-base titration with benzoic acid, the weighing as sulphate and the weighing as carbonai c. -
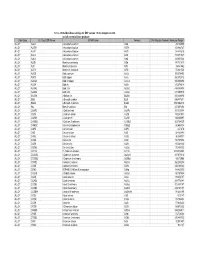
Database Full Listing
16-Nov-06 OLI Data Base Listings for ESP version 7.0.46, Analyzers 2.0.46 and all current alliance products Data Base OLI Tag (ESP) Name IUPAC Name Formula CAS Registry Number Molecular Weight ALLOY AL2U 2-Aluminum uranium Al2U 291.98999 ALLOY AL3TH 3-Aluminum thorium Al3Th 312.982727 ALLOY AL3TI 3-Aluminum titanium Al3Ti 128.824615 ALLOY AL3U 3-Aluminum uranium Al3U 318.971527 ALLOY AL4U 4-Aluminum uranium Al4U 345.953064 ALLOY ALSB Aluminum antimony AlSb 148.731537 ALLOY ALTI Aluminum titanium AlTi 74.861542 ALLOY ALTI3 Aluminum 3-titanium AlTi3 170.621536 ALLOY AUCD Gold cadmium AuCd 309.376495 ALLOY AUCU Gold copper AuCu 260.512512 ALLOY AUCU3 Gold 3-copper AuCu3 387.604492 ALLOY AUSN Gold tin AuSn 315.676514 ALLOY AUSN2 Gold 2-tin AuSn2 434.386505 ALLOY AUSN4 Gold 4-tin AuSn4 671.806519 ALLOY BA2SN 2-Barium tin Ba2Sn 393.369995 ALLOY BI2U 2-Bismuth uranium Bi2U 655.987671 ALLOY BI4U3 4-Bismuth 3-uranium Bi4U3 1550.002319 ALLOY BIU Bismuth uranium BiU 447.007294 ALLOY CA2PB 2-Calcium lead Ca2Pb 287.355988 ALLOY CA2SI 2-Calcium silicon Ca2Si 108.241501 ALLOY CA2SN 2-Calcium tin Ca2Sn 198.865997 ALLOY CA3SB2 3-Calcium 2-antimony Ca3Sb2 363.734009 ALLOY CAMG2 Calcium 2-magnesium CaMg2 88.688004 ALLOY CAPB Calcium lead CaPb 247.278 ALLOY CASI Calcium silicon CaSi 68.163498 ALLOY CASI2 Calcium 2-silicon CaSi2 96.249001 ALLOY CASN Calcium tin CaSn 158.787994 ALLOY CAZN Calcium zinc CaZn 105.468002 ALLOY CAZN2 Calcium 2-zinc CaZn2 170.858002 ALLOY CD11U 11-Cadmium uranium Cd11U 1474.536865 ALLOY CD3AS2 3-Cadmium 2-arsenic As2Cd3 487.073212 -

Galaxy Resources: Mt. Cattlin (Western Australia) Project No. 2541 NI43-101 Technical Report 31 December 2011
Final Galaxy Resources: Mt. Cattlin (Western Australia) Project No. 2541 NI43-101 Technical Report 31 December 2011 Office Locations This report has been prepared by Snowden Mining Industry Consultants („Snowden‟) on behalf of Galaxy Resources. Perth 87 Colin St, West Perth WA 6005 2011 AUSTRALIA PO Box 77, West Perth WA 6872 All rights are reserved. No part of this document may be reproduced, AUSTRALIA stored in a retrieval system, or transmitted in any form or by any means, Tel: +61 8 9213 9213 electronic, mechanical, photocopying, recording or otherwise, without the Fax: +61 8 9322 2576 prior written permission of Snowden. ABN: 99 085 319 562 [email protected] Brisbane Level 15, 300 Adelaide Street Prepared Robert Spiers Brisbane QLD 4000 AUSTRALIA by BSc, (Hons - DMajor Geology / Geophysics), MAIG Senior Consultant - Resource Geology .............................................................. PO Box 2207, Brisbane QLD 4001 AUSTRALIA Jeremy Peters BEng (Mining Engineering), Tel: +61 7 3231 3800 BSc, (Geology), Mine Managers Certificate, MAusIMM Fax: +61 7 3211 9815 Principal Consultant – Mining ............................................................................. ABN: 99 085 319 562 [email protected] Leon Lorenzen PhD (Metallurgical Engineering), MSc(Eng), BEng (Chemical Engineering), PrEng, Johannesburg CPEng, CEng, PrEng, FAusIMM, FSAIMM, Technology House, Greenacres Office FIChemE, FSAAE Park, Cnr. Victory and Rustenburg Group General Manager / Executive Consultant (Metallurgy) ............................. Roads, Victory Park JOHANNESBURG 2195 SOUTH AFRICA PO Box 2613, Parklands 2121 SOUTH AFRICA Tel: + 27 11 782 2379 Fax: + 27 11 782 2396 Reg No. 1998/023556/07 [email protected] Vancouver Suite 550, 1090 West Pender St, VANCOUVER BC V6E 2N7 CANADA Tel: +1 604 683 7645 Fax: +1 604 683 7929 Reg No. -

(12) United States Patent (10) Patent No.: US 8,741,256 B1 Harrison (45) Date of Patent: Jun
USOO874.1256B1 (12) United States Patent (10) Patent No.: US 8,741,256 B1 Harrison (45) Date of Patent: Jun. 3, 2014 (54) PREPARATION OF LITHIUM CARBONATE 4,036,713 A 7, 1977 Brown FROM LITHIUM CHILORIDE CONTAINING 4,142,950 A * 3/1979 Creamer et al. .............. 205,531 BRINES 4,209,369 A 6, 1980 Seko et al. 5,219,550 A 6/1993 Brown et al. 5,919.287 A * 7/1999 Moreau ........................... 95/130 (75) Inventor: Stephen Harrison, Benicia, CA (US) 5,951,843. A 9, 1999 Itoh et al. 5,993,759 A * 1 1/1999 Wilkomirsky ............. 423,179.5 (73) Assignee: Simbol Inc., Pleasanton, CA (US) 7,708,972 B2 5/2010 Coustry et al. 2005.0011753 A1 1/2005 Jackson et al. (*) Notice: Subject to any disclaimer, the term of this 2007/01 14134 A1* 5/2007 Legget al. .................... 205/.338 patent is extended or adjusted under 35 U.S.C. 154(b) by 409 days. FOREIGN PATENT DOCUMENTS EP 1900688 A1 * 3, 2008 ............... COD 707 (21) Appl. No.: 12/766,589 WO 2009131628 A1 10, 2009 (22) Filed: Apr. 23, 2010 OTHER PUBLICATIONS European Search Report and Written Opinion, Aug. 14, 2013. Related U.S. Application Data p p p 9. (60) Provisional application No. 61/172,540, filed on Apr. * cited by examiner 24, 2009. Primary Examiner — Stanley Silverman (51) Int. Cl. Assistant Examiner — Richard M. Rump COID 7700 (2006.01) (74) Attorney, Agent, or Firm — Bracewell & Giuliani LLP: (52) U.S. Cl. Michael R. Samardzija USPC ........................................... 423/421; 423/427 (57) ABSTRACT (58) USPCField of ................................................. -

Process for Preparing Highly Pure Lithium Carbonate
(19) TZZ ¥¥_T (11) EP 2 536 663 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C01D 15/08 (2006.01) C01B 25/10 (2006.01) 26.03.2014 Bulletin 2014/13 C01D 15/04 (2006.01) C01D 15/02 (2006.01) C01B 25/45 (2006.01) C01B 25/30 (2006.01) (2006.01) (2006.01) (21) Application number: 11707259.5 C07F 1/02 C01B 11/18 C01B 35/06 (2006.01) C07F 5/02 (2006.01) C25B 1/02 (2006.01) C25B 3/00 (2006.01) (22) Date of filing: 17.02.2011 (86) International application number: PCT/US2011/025256 (87) International publication number: WO 2011/103298 (25.08.2011 Gazette 2011/34) (54) PROCESS FOR PREPARING HIGHLY PURE LITHIUM CARBONATE VERFAHREN FÜR DIE ZUBEREITUNG VON HOCHREINEM LITHIUMCARBONAT PROCÉDÉ POUR LA PRÉPARATION DE CARBONATE DE LITHIUM DE HAUTE PURETÉ (84) Designated Contracting States: (74) Representative: Barton, Matthew Thomas AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Forresters GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Skygarden PL PT RO RS SE SI SK SM TR Erika-Mann-Strasse 11 80636 München (DE) (30) Priority: 17.02.2010 US 305213 P (56) References cited: (43) Date of publication of application: WO-A1-99/29624 DE-A1- 19 809 420 26.12.2012 Bulletin 2012/52 GB-A- 2 190 668 US-A1- 2006 115 396 (73) Proprietor: Simbol , Inc. • DATABASE WPI Week 200512 Thomson Pleasanton CA 94566 (US) Scientific, London, GB; AN 2005-109280 XP002666413, & RU 2 243 157 C2 (NOVOS CHEM (72) Inventors: CONCENTRATES STOCK CO) 27 December 2004 • HARRISON, Stephen (2004-12-27) Benicia, CA (US) • BLANCHET, Robert Palm Desert, CA (CA) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations.