Arginine:Glycine Amidinotransferase Deficiency
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CCA One Care Options Formulary
Commonwealth Care Alliance One Care Plan (Medicare-Medicaid Plan) 2021 List of Covered Drugs (Formulary) 30 Winter Street • Boston, MA 02108 PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN For more recent information or other questions, contact Commonwealth Care Alliance Member Services at 1-866-610-2273 (TTY: call MassRelay at 711), 8 a.m. – 8 p.m., 7 days a week, or visit www.commonwealthonecare.org H0137_CF2021 Approved Formulary: ID 00021588 • Version 13 • Updated on 08/01/2021 One Care Plan | 2021 List of Covered Drugs (Formulary) Introduction This document is called the List of Covered Drugs (also known as the Drug List). It tells you which prescription drugs, over-the-counter drugs and items are covered by Commonwealth Care Alliance. The Drug List also tells you if there are any special rules or restrictions on any drugs covered by One Care. Key terms and their definitions appear in the last chapter of the Member Handbook. Table of Contents A. Disclaimers ........................................................................................................................ 4 B. Frequently Asked Questions (FAQ) .................................................................................. 5 What prescription drugs are on the List of Covered Drugs? (We call the List of Covered Drugs the “Drug List” for short.) ................................................................... 5 B2. Does the Drug List ever change? ............................................................................... 5 B3. What happens when there is a change to the Drug List? ........................................... 6 B4. Are there any restrictions or limits on drug coverage or any required actions to take to get certain drugs? .................................................................................................. 7 B5. How will you know if the drug you want has limitations or if there are required actions to take to get the drug? ................................................................................. -

Amino Acid Recognition by Aminoacyl-Trna Synthetases
www.nature.com/scientificreports OPEN The structural basis of the genetic code: amino acid recognition by aminoacyl‑tRNA synthetases Florian Kaiser1,2,4*, Sarah Krautwurst3,4, Sebastian Salentin1, V. Joachim Haupt1,2, Christoph Leberecht3, Sebastian Bittrich3, Dirk Labudde3 & Michael Schroeder1 Storage and directed transfer of information is the key requirement for the development of life. Yet any information stored on our genes is useless without its correct interpretation. The genetic code defnes the rule set to decode this information. Aminoacyl-tRNA synthetases are at the heart of this process. We extensively characterize how these enzymes distinguish all natural amino acids based on the computational analysis of crystallographic structure data. The results of this meta-analysis show that the correct read-out of genetic information is a delicate interplay between the composition of the binding site, non-covalent interactions, error correction mechanisms, and steric efects. One of the most profound open questions in biology is how the genetic code was established. While proteins are encoded by nucleic acid blueprints, decoding this information in turn requires proteins. Te emergence of this self-referencing system poses a chicken-or-egg dilemma and its origin is still heavily debated 1,2. Aminoacyl-tRNA synthetases (aaRSs) implement the correct assignment of amino acids to their codons and are thus inherently connected to the emergence of genetic coding. Tese enzymes link tRNA molecules with their amino acid cargo and are consequently vital for protein biosynthesis. Beside the correct recognition of tRNA features3, highly specifc non-covalent interactions in the binding sites of aaRSs are required to correctly detect the designated amino acid4–7 and to prevent errors in biosynthesis5,8. -

Functional Characterization of BC039389-GATM and KLK4
Pflueger et al. BMC Genomics (2015) 16:247 DOI 10.1186/s12864-015-1446-z RESEARCH ARTICLE Open Access Functional characterization of BC039389-GATM and KLK4-KRSP1 chimeric read-through transcripts which are up-regulated in renal cell cancer Dorothee Pflueger1,2, Christiane Mittmann1, Silvia Dehler3, Mark A Rubin4,5, Holger Moch1,2 and Peter Schraml1* Abstract Background: Chimeric read-through RNAs are transcripts originating from two directly adjacent genes (<10 kb) on the same DNA strand. Although they are found in next-generation whole transcriptome sequencing (RNA-Seq) data on a regular basis, investigating them further has usually been refrained from. Therefore, their expression patterns or functions in general, and in oncogenesis in particular, are poorly understood. Results: We used paired-end RNA-Seq and a specifically designed computational data analysis pipeline (FusionSeq) to nominate read-through events in a small discovery set of renal cell carcinomas (RCC) and confirmed them in a larger validation cohort. 324 read-through events were called overall; 22/27 (81%) selected nominees passed validation with conventional PCR and were sequenced at the junction region. We frequently identified various isoforms of a given read-through event. 2/22 read-throughs were up-regulated: BC039389-GATM was higher expressed in RCC compared to benign adjacent kidney; KLK4-KRSP1 was expressed in 46/169 (27%) RCCs, but rarely in normal tissue. KLK4-KRSP1 expression was associated with worse clinical outcome in the patient cohort. In cell lines, both read-throughs influenced molecular mechanisms (i.e. target gene expression or migration/invasion) in a way that counteracted the effect of the respective parent transcript GATM or KLK4. -

Laboratory Diagnosis of Creatine Deficiency Syndromes: a Technical Standard and Guideline of the American College of Medical Genetics and Genomics
ACMG STaNDaRDs aND GUIDELINEs © American College of Medical Genetics and Genomics Laboratory diagnosis of creatine deficiency syndromes: a technical standard and guideline of the American College of Medical Genetics and Genomics J. Daniel Sharer, PhD1, Olaf Bodamer, MD, PhD2, Nicola Longo, MD, PhD3, Silvia Tortorelli, MD, PhD4,5, Mirjam M.C. Wamelink, PhD6 and Sarah Young, PhD7; a Workgroup of the ACMG Laboratory Quality Assurance Committee Disclaimer: These ACMG Standards and Guidelines are intended as an educational resource for clinical laboratory geneticists to help them provide quality clinical laboratory genetic services. Adherence to these standards and guidelines is voluntary and does not necessarily assure a successful medical outcome. These Standards and Guidelines should not be considered inclusive of all proper procedures and tests or exclusive of others that are reasonably directed to obtaining the same results. In determining the propriety of any specific procedure or test, clinical laboratory geneticists should apply their professional judgment to the specific circumstances presented by the patient or specimen. Clinical laboratory geneticists are encouraged to document in the patient’s record the rationale for the use of a particular procedure or test, whether or not it is in conformance with these Standards and Guidelines. They also are advised to take notice of the date any particular guideline was adopted, and to consider other relevant medical and scientific information that becomes available after that date. It also would be prudent to consider whether intellectual property interests may restrict the performance of certain tests and other procedures. Cerebral creatine deficiency syndromes are neurometabolic condi- the diagnosis of creatine deficiency syndromes. -

Chuanxiong Rhizoma Compound on HIF-VEGF Pathway and Cerebral Ischemia-Reperfusion Injury’S Biological Network Based on Systematic Pharmacology
ORIGINAL RESEARCH published: 25 June 2021 doi: 10.3389/fphar.2021.601846 Exploring the Regulatory Mechanism of Hedysarum Multijugum Maxim.-Chuanxiong Rhizoma Compound on HIF-VEGF Pathway and Cerebral Ischemia-Reperfusion Injury’s Biological Network Based on Systematic Pharmacology Kailin Yang 1†, Liuting Zeng 1†, Anqi Ge 2†, Yi Chen 1†, Shanshan Wang 1†, Xiaofei Zhu 1,3† and Jinwen Ge 1,4* Edited by: 1 Takashi Sato, Key Laboratory of Hunan Province for Integrated Traditional Chinese and Western Medicine on Prevention and Treatment of 2 Tokyo University of Pharmacy and Life Cardio-Cerebral Diseases, Hunan University of Chinese Medicine, Changsha, China, Galactophore Department, The First 3 Sciences, Japan Hospital of Hunan University of Chinese Medicine, Changsha, China, School of Graduate, Central South University, Changsha, China, 4Shaoyang University, Shaoyang, China Reviewed by: Hui Zhao, Capital Medical University, China Background: Clinical research found that Hedysarum Multijugum Maxim.-Chuanxiong Maria Luisa Del Moral, fi University of Jaén, Spain Rhizoma Compound (HCC) has de nite curative effect on cerebral ischemic diseases, *Correspondence: such as ischemic stroke and cerebral ischemia-reperfusion injury (CIR). However, its Jinwen Ge mechanism for treating cerebral ischemia is still not fully explained. [email protected] †These authors share first authorship Methods: The traditional Chinese medicine related database were utilized to obtain the components of HCC. The Pharmmapper were used to predict HCC’s potential targets. Specialty section: The CIR genes were obtained from Genecards and OMIM and the protein-protein This article was submitted to interaction (PPI) data of HCC’s targets and IS genes were obtained from String Ethnopharmacology, a section of the journal database. -

Inherited Renal Tubulopathies—Challenges and Controversies
G C A T T A C G G C A T genes Review Inherited Renal Tubulopathies—Challenges and Controversies Daniela Iancu 1,* and Emma Ashton 2 1 UCL-Centre for Nephrology, Royal Free Campus, University College London, Rowland Hill Street, London NW3 2PF, UK 2 Rare & Inherited Disease Laboratory, London North Genomic Laboratory Hub, Great Ormond Street Hospital for Children National Health Service Foundation Trust, Levels 4-6 Barclay House 37, Queen Square, London WC1N 3BH, UK; [email protected] * Correspondence: [email protected]; Tel.: +44-2381204172; Fax: +44-020-74726476 Received: 11 February 2020; Accepted: 29 February 2020; Published: 5 March 2020 Abstract: Electrolyte homeostasis is maintained by the kidney through a complex transport function mostly performed by specialized proteins distributed along the renal tubules. Pathogenic variants in the genes encoding these proteins impair this function and have consequences on the whole organism. Establishing a genetic diagnosis in patients with renal tubular dysfunction is a challenging task given the genetic and phenotypic heterogeneity, functional characteristics of the genes involved and the number of yet unknown causes. Part of these difficulties can be overcome by gathering large patient cohorts and applying high-throughput sequencing techniques combined with experimental work to prove functional impact. This approach has led to the identification of a number of genes but also generated controversies about proper interpretation of variants. In this article, we will highlight these challenges and controversies. Keywords: inherited tubulopathies; next generation sequencing; genetic heterogeneity; variant classification. 1. Introduction Mutations in genes that encode transporter proteins in the renal tubule alter kidney capacity to maintain homeostasis and cause diseases recognized under the generic name of inherited tubulopathies. -

Amino Acid Chemistry
Handout 4 Amino Acid and Protein Chemistry ANSC 619 PHYSIOLOGICAL CHEMISTRY OF LIVESTOCK SPECIES Amino Acid Chemistry I. Chemistry of amino acids A. General amino acid structure + HN3- 1. All amino acids are carboxylic acids, i.e., they have a –COOH group at the #1 carbon. 2. All amino acids contain an amino group at the #2 carbon (may amino acids have a second amino group). 3. All amino acids are zwitterions – they contain both positive and negative charges at physiological pH. II. Essential and nonessential amino acids A. Nonessential amino acids: can make the carbon skeleton 1. From glycolysis. 2. From the TCA cycle. B. Nonessential if it can be made from an essential amino acid. 1. Amino acid "sparing". 2. May still be essential under some conditions. C. Essential amino acids 1. Branched chain amino acids (isoleucine, leucine and valine) 2. Lysine 3. Methionine 4. Phenyalanine 5. Threonine 6. Tryptophan 1 Handout 4 Amino Acid and Protein Chemistry D. Essential during rapid growth or for optimal health 1. Arginine 2. Histidine E. Nonessential amino acids 1. Alanine (from pyruvate) 2. Aspartate, asparagine (from oxaloacetate) 3. Cysteine (from serine and methionine) 4. Glutamate, glutamine (from α-ketoglutarate) 5. Glycine (from serine) 6. Proline (from glutamate) 7. Serine (from 3-phosphoglycerate) 8. Tyrosine (from phenylalanine) E. Nonessential and not required for protein synthesis 1. Hydroxyproline (made postranslationally from proline) 2. Hydroxylysine (made postranslationally from lysine) III. Acidic, basic, polar, and hydrophobic amino acids A. Acidic amino acids: amino acids that can donate a hydrogen ion (proton) and thereby decrease pH in an aqueous solution 1. -

Amino Acids Amino Acids
Amino Acids Amino Acids What Are Amino Acids? Essential Amino Acids Non Essential Amino Acids Amino acids are the building blocks of proteins; proteins are made of amino acids. Isoleucine Arginine (conditional) When you ingest a protein your body breaks it down into the individual aminos, Leucine Glutamine (conditional) reorders them, re-folds them, and turns them into whatever is needed by the body at Lysine Tyrosine (conditional) that time. From only 20 amino acids, the body is able to make thousands of unique proteins with different functions. Methionine Cysteine (conditional) Phenylalanine Glycine (conditional) Threonine Proline (conditional) Did You Know? Tryptophan Serine (conditional) Valine Ornithine (conditional) There are 20 different types of amino acids that can be combined to make a protein. Each protein consists of 50 to 2,000 amino acids that are connected together in a specific Histidine* Alanine sequence. The sequence of the amino acids determines each protein’s unique structure Asparagine and its specific function in the body. Asparate Popular Amino Acid Supplements How Do They Benefit Our Health? Acetyl L- Carnitine: As part of its role in supporting L-Lysine: L-Lysine, an essential amino acid, is mental function, Acetyl L-Carnitine may help needed to support proper growth and bone Proteins (amino acids) are needed by your body to maintain muscles, bones, blood, as support memory, attention span and mental development. It can also support immune function. well as create enzymes, neurotransmitters and antibodies, as well as transport and performance. store molecules. N-Acetyl Cysteine: N-Acetyl Cysteine (NAC) is a L-Arginine: L-Arginine is a nonessential amino acid form of the amino acid cysteine. -

Stimulation Effects of Foliar Applied Glycine and Glutamine Amino Acids
Open Agriculture. 2019; 4: 164–172 Research Article Yaghoub Aghaye Noroozlo, Mohammad Kazem Souri*, Mojtaba Delshad Stimulation Effects of Foliar Applied Glycine and Glutamine Amino Acids on Lettuce Growth https://doi.org/10.1515/opag-2019-0016 received June 27, 2018; accepted January 20, 2019 1 Introduction Abstract: Amino acids have various roles in plant In biology, amino acids have vital roles in cell life. Amino metabolism, and exogenous application of amino acids acids are among the most important primary metabolites may have benefits and stimulation effects on plant growth within the plant cells. However, they are frequently and quality. In this study, the growth and nutrient uptake regarded as secondary metabolites, particularly in the of Romain lettuce (Lactuca sativa subvar Sahara) were case of proline, glycine and betaine amino acids. Many evaluated under spray of glycine or glutamine at different physiochemical characteristics of plant cells, tissues and concentrations of 0 (as control), 250, 500 and 1000 organs are influenced by the presence of amino acids (Rai mg.L-1, as well as a treatment of 250 mg.L-1 glycine+250 2002; Marschner 2011). They are the building units of mg.L-1 glutamine. The results showed that there was proteins, as the main component of living cells that have significant increase in leaf total chlorophyll content under vital roles in many cell metabolic reactions (Kielland 1994; Gly250+Glu250, Gly250 and Glu1000 mg.L-1treatments, and Rainbird et al. 1984; Jones and Darrah 1993). In addition, in leaf carotenoids content under 250 mg.L-1 glutamine amino acids have various important biological functions spray compared with the control plants. -

List of Toxic Chemicals Within the Glycol Ethers Category
United States Office of Environmental Revised December 2000 Environmental Protection Information EPA 745-R-00-004 Agency Washington, DC 20460 TOXICS RELEASE INVENTORY List of Toxic Chemicals within the Glycol Ethers Category Section 313 of the Emergency Planning and Community Right-to-Know Act (EPCRA) requires certain facilities manufacturing, processing, or otherwise using listed toxic chemicals to report their environmental releases of such chemicals annually. Beginning with the 1991 reporting year, such facilities also must report pollution prevention and recycling data for such chemicals, pursuant to section 6607 of the Pollution Prevention Act, 42 U.S.C. 13106. When enacted, EPCRA section 313 established an initial list of toxic chemicals that was comprised of more than 300 chemicals and 20 chemical categories. EPCRA section 313(d) authorizes EPA to add chemicals to or delete chemicals from the list, and sets forth criteria for these actions. CONTENTS Section 1. Introduction ...................................................... 3 Section 2. CAS Number List of Some Chemicals within the Glycol Ethers Category ........ 6 Section 3. CAS Number List of Some Mixtures That Contain Glycol Ethers within the Category .............................................. 185 Section 4. CAS Number List of Some Oligomeric or Polymeric Chemicals That Might Contain Glycol Ether Components within the Category .......................... 187 FOREWORD This document is an updated version of the previous document, EPA 745-R-99-006, June 1999. This version has the following updates: • The titles to Table 1 on page 6, Table 2 on page 185, and Table 3 on 187 are modified; and • The CAS number of second listing in Table 3 (Poly(oxy-1,2-ethanediyl), .alpha.- (phenylsulfonyl)-.omega.-methoxy-) on page 187 is changed from 7664-41-7 to 67584-43-4. -

Nucleotide Base Coding and Am1ino Acid Replacemients in Proteins* by Emil L
VOL. 48, 1962 BIOCHEMISTRY: E. L. SAIITH 677 18 Britten, R. J., and R. B. Roberts, Science, 131, 32 (1960). '9 Crestfield, A. M., K. C. Smith, and F. WV. Allen, J. Biol. Chem., 216, 185 (1955). 20 Gamow, G., Nature, 173, 318 (1954). 21 Brenner, S., these PROCEEDINGS, 43, 687 (1957). 22 Nirenberg, M. WV., J. H. Matthaei, and 0. WV. Jones, unpublished data. 23 Crick, F. H. C., L. Barnett, S. Brenner, and R. J. Watts-Tobin, Nature, 192, 1227 (1961). 24 Levene, P. A., and R. S. Tipson, J. Biol. Ch-nn., 111, 313 (1935). 25 Gierer, A., and K. W. Mundry, Nature, 182, 1437 (1958). 2' Tsugita, A., and H. Fraenkel-Conrat, J. Mllot. Biol., in press. 27 Tsugita, A., and H. Fraenkel-Conrat, personal communication. 28 Wittmann, H. G., Naturwissenschaften, 48, 729 (1961). 29 Freese, E., in Structure and Function of Genetic Elements, Brookhaven Symposia in Biology, no. 12 (1959), p. 63. NUCLEOTIDE BASE CODING AND AM1INO ACID REPLACEMIENTS IN PROTEINS* BY EMIL L. SMITHt LABORATORY FOR STUDY OF HEREDITARY AND METABOLIC DISORDERS AND THE DEPARTMENTS OF BIOLOGICAL CHEMISTRY AND MEDICINE, UNIVERSITY OF UTAH COLLEGE OF MEDICINE Communicated by Severo Ochoa, February 14, 1962 The problem of which bases of messenger or template RNA' specify the coding of amino acids in proteins has been largely elucidated by the use of synthetic polyri- bonucleotides.2-7 For these triplet nucleotide compositions (Table 1), it is of in- terest to examine some of the presently known cases of amino acid substitutions in polypeptides or proteins of known structure. -
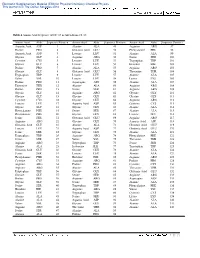
Table 2 Amino Acid Sequence of OC-17 As Taken from Ref. 28 Amino
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics This journal is © The Owner Societies 2012 Table 2 Amino Acid Sequence of OC-17 as taken from ref. 28 Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Aspartic Acid ASP 1 Alanine ALA 49 Arginine ARG 97 Proline PRO 2 Glutamic Acid GLU 50 Phenyalanine PHE 98 Aspartic Acid ASP 3 Leucine LEU 51 Alanine ALA 99 Glycine GLY 4 Arginine ARG 52 Serine SER 100 Cysteine CYS 5 Leucine LEU 53 Tryptophan TRP 101 Glycine GLY 6 Leucine LEU 54 Histidine HIE 102 Proline PRO 7 Alanine ALA 55 Arginine ARG 103 Glycine GLY 8 Glutamic Acid GLU 56 Threonine THR 104 Tryptophan TRP 9 Leucine LEU 57 Alanine ALA 105 Valine VAL 10 Leucine LEU 58 Lysine LYS 106 Proline PRO 11 Asparagine ASN 59 Alanine ALA 107 Threonine THR 12 Alanine ALA 60 Arginine ARG 108 Proline PRO 13 Serine SER 61 Arginine ARG 109 Glycine GLY 14 Arginine ARG 62 Glycine GLY 110 Glycine GLY 15 Glycine GLY 63 Glycine GLY 111 Cysteine CYS 16 Glycine GLY 64 Arginine ARG 112 Leucine LEU 17 Aspartic Acid ASP 65 Cysteine CYS 113 Glycine GLY 18 Glycine GLY 66 Alanine ALA 114 Phenyalanine PHE 19 Serine SER 67 Alanine ALA 115 Phenyalanine PHE 20 Glycine GLY 68 Leucine LEU 116 Serine SER 21 Glutamic Acid GLU 69 Arginine ARG 117 Arginine ARG 22 Glycine GLY 70 Aspartic Acid ASP 118 Glutamic Acid GLU 23 Alanine ALA 71 Glutamic Acid GLU 119 Leucine LEU 24 Aspartic Acid ASP 72 Glutamic Acid GLU 120 Serine SER 25 Glycine GLY 73 Alanine ALA 121 Tryptophan TRP 26 Arginine ARG 74 Phenyalanine