Avoid Clichés Like…
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

George De Hevesy in America
Journal of Nuclear Medicine, published on July 13, 2019 as doi:10.2967/jnumed.119.233254 George de Hevesy in America George de Hevesy was a Hungarian radiochemist who was awarded the Nobel Prize in Chemistry in 1943 for the discovery of the radiotracer principle (1). As the radiotracer principle is the foundation of all diagnostic and therapeutic nuclear medicine procedures, Hevesy is widely considered the father of nuclear medicine (1). Although it is well-known that he spent time at a number of European institutions, it is not widely known that he also spent six weeks at Cornell University in Ithaca, NY, in the fall of 1930 as that year’s Baker Lecturer in the Department of Chemistry (2-6). “[T]he Baker Lecturer gave two formal presentations per week, to a large and diverse audience and provided an informal seminar weekly for students and faculty members interested in the subject. The lecturer had an office in Baker Laboratory and was available to faculty and students for further discussion.” (7) There is also evidence that, “…Hevesy visited Harvard [University, Cambridge, MA] as a Baker Lecturer at Cornell in 1930…” (8). Neither of the authors of this Note/Letter was aware of Hevesy’s association with Cornell University despite our longstanding ties to Cornell until one of us (WCK) noticed the association in Hevesy’s biographical page on the official Nobel website (6). WCK obtained both his undergraduate degree and medical degree from Cornell in Ithaca and New York City, respectively, and spent his career in nuclear medicine. JRO did his nuclear medicine training at Columbia University and has subsequently been a faculty member of Weill Cornell Medical College for the last eleven years (with a brief tenure at Memorial Sloan Kettering Cancer Center (affiliated with Cornell)), and is now the program director of the Nuclear Medicine residency and Chief of the Molecular Imaging and Therapeutics Section. -

Otto Hahn Otto Hahn
R.N. 70269/98 Postal Registration No.: DL-SW-1/4082/15-17 ISSN : 0972-169X Date of posting: 26-27 of advance month Date of publication: 24 of advance month May 2017 Vol. 19 No. 8 Rs. 5.00 Otto Hahn Discoverer of Nuclear Fission Editorial: Consolidating 35 science communication activities in our country Otto Hahn: Discoverer of 34 Nuclear Fission Keep Your Eyes Healthy 31 Phenol: A Serious 30 Environmental Threat Accidental Discoveries in 28 Medical Science Cures for haemorrhoids— 24 Simple treatments and Surgeries Recent developments 21 in science and technology 36 Editorial Consolidating science communication activities in our country Dr. R. Gopichandran It is well known that the National Council of Science Museums of India’s leadership in science technology and innovation (STI) across the Ministry of Culture, Government of India, the National Institute the bilateral and multilateral framework also. The news feature service of Science Communication and Information Resources (NISCAIR) and the portal activity have well defined action plans to reach out to of CSIR, the National Council for Science and Technology fellow institutions and citizens with suitably embellished platform Communication (NCSTC) of the Department of Science and and opportunities for all to deliver together. Technology (DST), Government of India and Vigyan Prasar, also While these are interesting and extremely important, especially of DST, have been carrying out excellent science communication because they respond to the call to upscale and value add science activities over the years. It cannot be denied that the reach has been and technology communication, it is equally important to document quite significant collectively. -

Appendix E Nobel Prizes in Nuclear Science
Nuclear Science—A Guide to the Nuclear Science Wall Chart ©2018 Contemporary Physics Education Project (CPEP) Appendix E Nobel Prizes in Nuclear Science Many Nobel Prizes have been awarded for nuclear research and instrumentation. The field has spun off: particle physics, nuclear astrophysics, nuclear power reactors, nuclear medicine, and nuclear weapons. Understanding how the nucleus works and applying that knowledge to technology has been one of the most significant accomplishments of twentieth century scientific research. Each prize was awarded for physics unless otherwise noted. Name(s) Discovery Year Henri Becquerel, Pierre Discovered spontaneous radioactivity 1903 Curie, and Marie Curie Ernest Rutherford Work on the disintegration of the elements and 1908 chemistry of radioactive elements (chem) Marie Curie Discovery of radium and polonium 1911 (chem) Frederick Soddy Work on chemistry of radioactive substances 1921 including the origin and nature of radioactive (chem) isotopes Francis Aston Discovery of isotopes in many non-radioactive 1922 elements, also enunciated the whole-number rule of (chem) atomic masses Charles Wilson Development of the cloud chamber for detecting 1927 charged particles Harold Urey Discovery of heavy hydrogen (deuterium) 1934 (chem) Frederic Joliot and Synthesis of several new radioactive elements 1935 Irene Joliot-Curie (chem) James Chadwick Discovery of the neutron 1935 Carl David Anderson Discovery of the positron 1936 Enrico Fermi New radioactive elements produced by neutron 1938 irradiation Ernest Lawrence -

4. Marie Curie, Ethics and Research
CATHERINE MILNE 4. MARIE CURIE, ETHICS AND RESEARCH INTRODUCTION Recently Chemistry and Engineering News (Bard, Prestwich, Wight, Heller, & Zimmerman, 2010) carried a series of commentaries on the culture of academic research in chemistry with a focus on the role of funding in research. Initially, Alan Bard decried how decisions about tenure seem more and more to focus on grant getting rather than consideration of the applicant’s accomplishments generated because of access to this funding. Bard argued further that often this funding was based, not on the quality of the proposed research but on a researcher’s ability to “hype their research” and damming truth in the process (Bard et al., 2010, p. 27). According to him, there was a disturbing trend in universities for researchers to be encouraged, almost expected, to generate patents and from there, even to be involved in initiating “start up” companies. Other researchers responded. Glen Prestwich and Charles Wight (Bard et al., 2010) argue that if researchers were able to present a clear connection between “taxpayer dollars” and funded research, the public would realize the value that science delivers. Prestwich and Wight argue further that a new cadre of researchers is involved in identifying real world problems, translating basic research into applications, and creating products as well as publications. Moving from basic research to applications that address real world problems is called translational research, and Prestwich and Wight see this research as a positive development in chemistry. Adam Heller (Bard et al., 2010) weighs in claiming that the pursuit of “patent-protected, people-serving products” was not “a sad sign of our times but a reawakening of the proud history of academic chemistry and chemical engineering” (p. -

Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production. by Vaclav Smil. Cambridge: MIT Press, 2004
Book Reviews / 383 nessing water power to produce scythes for farmers, spindles for textile mills, and cotton thread. Small emphasizes that these were not the activities of ab- sentee investors but of local men and women who mixed manufacturing with agriculture. Beauty and Convenience focuses on a farming community that has received scant attention from historians, but Small makes a convincing case that the way in which the farmers of Sutton fashioned and re-fashioned their houses and landscape reveals their full participation in the historic transformations of their era. Kenneth Hafertepe Baylor University Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production. By Vaclav Smil. Cambridge: MIT Press, 2004. 360 pp., $19.95, paperback, ISBN 0-262-69313-5. What is the most important technological invention in the twentieth century? Vaclav Smil makes the cogent argument that it is the discovery and industrial- ization of ammonia synthesis by Fritz Haber and Carl Bosch. Without indus- trial N-fixation, as much as two-fifths of the world’s population would starve for want of available protein in their diet, and major advances in high-pressure industrial processes would have been delayed. Likewise, the Haber-Bosch process facilitated world munitions production; encouraged agricultural ex- pansion onto marginal lands, leading to greater greenhouse emissions and soil degradation; and (because of inherent inefficiencies in nitrogen recovery by plants) has led to an unbalanced enrichment of natural ecosystems by nitrogen. It is an odd book given the title. History and society would be unalterably different without Haber and Bosch, but these protagonists do not appear un- til chapters four and five, respectively; then their lives are described in the most perfunctory manner. -

Recollections and Reconstructions
@ ? Editor's Note: The Society's Nuclear Pioneer Citationfor 1977 goes not to an individual but to a distinguished group of individuals: those who took part in the epochal “ChicagoPile―experiment of Dec. 2, 1942, which produced thefirst successful atomicpile chain reaction. The 42 members ofthe Chicago Pilegroup are listed on page 591. One ofthose named, Harold M. Agnew, now Directorofthe Los Alamos Scient@flc Laboratory, is the Nuclear Pioneer Lecturerfor the 24th Annual Meeting. The guidingforce behind the Chicago Pile experiment was Enrico Fermi, recipient of the Nuclear Pioneer Citation of 1963. In the artick which follows, Laura Fermi recreates the excitement and mystery that surrounded the work ofher husband's research team in the early 1940s in Chicago. @. The FirstAtomic Pile: Recollections and Reconstructions by Laura Fermi @ . @ / - .-i@ ‘@:@ Enrico FermIat work, ca. 1942. As far as I can remember at the distance ofalmost 35 Perhaps not all husbands adhered as strictly as years, the first guests to arrive at our party on that Enrico to the rules of secrecy when talking to their beastly cold December night were the Zinns. Wally wives. He couldn't have been more tight-lipped. Once! and Jean. As they shook the snow off their coats, related some gossip to him: “Peoplesay that you stamping their feet on the floor, Wally turned to scientists at the Met Lab are seeking a cure for Enrico and said: “Congratulations!― I was surprised; cancer. .““Arewe?―he asked with his usual for Enrico had given me no hint that anything unusual imperturbable expression. -

MIT-NSE by the 1930S It Was Time to Discover Fission. We Knew About Isoto
Prelude to the Manhattan Project Michael V. Hynes – MIT-NSE By the 1930s it was time to discover fission. We knew about isotopes. The Curies and Becquerel had discovered radioactivity. Rutherford had discovered the nucleus. Chadwick had discovered the neutron. Einstein had discovered special relativity and the famous equation E=mc2. Bethe was even publishing about fusion as the source of energy for the Sun. So, it was time. By the 1930s a human drama was unfolding in Europe and in the Pacific – the rise of fascism. The events of this drama engulfed the world in war and created a need on all sides for weaponry that could defeat the enemy by whatever means. The intersection of scientific inevitability and war created the environment for the advent of nuclear weapons. Science was practiced was very differently in the 1920s and 1930s than today. In that era science was highly specialized and compartmentalized. If you were a metallurgist for example it was unlikely that you would have anything to do with a physicist – that all changed in the1940s. In 1933 Leo Szilard (Fig. 1), a Hungarian physicist who took refuge in London from Nazi Germany, read a paper by Rutherford that ridiculed the idea of getting energy from nuclear transmutations. Szilard realized that if you could find an element which is split by neutrons and which would emit two neutrons in the process, then a chain reaction could be started. This is the basic idea of a nuclear weapon -- to generate energy from the chain reaction. Szilard was a chemist by training and knew about the idea of a chemical chain reaction. -
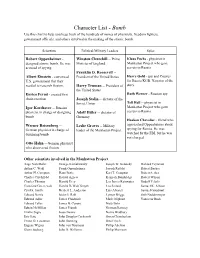
Character List
Character List - Bomb Use this chart to help you keep track of the hundreds of names of physicists, freedom fighters, government officials, and others involved in the making of the atomic bomb. Scientists Political/Military Leaders Spies Robert Oppenheimer - Winston Churchill -- Prime Klaus Fuchs - physicist in designed atomic bomb. He was Minister of England Manhattan Project who gave accused of spying. secrets to Russia Franklin D. Roosevelt -- Albert Einstein - convinced President of the United States Harry Gold - spy and Courier U.S. government that they for Russia KGB. Narrator of the needed to research fission. Harry Truman -- President of story the United States Enrico Fermi - created first Ruth Werner - Russian spy chain reaction Joseph Stalin -- dictator of the Tell Hall -- physicist in Soviet Union Igor Korchatov -- Russian Manhattan Project who gave physicist in charge of designing Adolf Hitler -- dictator of secrets to Russia bomb Germany Haakon Chevalier - friend who Werner Reisenberg -- Leslie Groves -- Military approached Oppenheimer about German physicist in charge of leader of the Manhattan Project spying for Russia. He was designing bomb watched by the FBI, but he was not charged. Otto Hahn -- German physicist who discovered fission Other scientists involved in the Manhattan Project: Aage Niels Bohr George Kistiakowsky Joseph W. Kennedy Richard Feynman Arthur C. Wahl Frank Oppenheimer Joseph Rotblat Robert Bacher Arthur H. Compton Hans Bethe Karl T. Compton Robert Serber Charles Critchfield Harold Agnew Kenneth Bainbridge Robert Wilson Charles Thomas Harold Urey Leo James Rainwater Rudolf Pelerls Crawford Greenewalt Harold DeWolf Smyth Leo Szilard Samuel K. Allison Cyril S. Smith Herbert L. Anderson Luis Alvarez Samuel Goudsmit Edward Norris Isidor I. -

Hitler's Uranium Club, the Secret Recordings at Farm Hall
HITLER’S URANIUM CLUB DER FARMHALLER NOBELPREIS-SONG (Melodie: Studio of seiner Reis) Detained since more than half a year Ein jeder weiss, das Unglueck kam Sind Hahn und wir in Farm Hall hier. Infolge splitting von Uran, Und fragt man wer is Schuld daran Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. The real reason nebenbei Die energy macht alles waermer. Ist weil we worked on nuclei. Only die Schweden werden aermer. Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Die nuclei waren fuer den Krieg Auf akademisches Geheiss Und fuer den allgemeinen Sieg. Kriegt Deutschland einen Nobel-Preis. Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Wie ist das moeglich, fragt man sich, In Oxford Street, da lebt ein Wesen, The story seems wunderlich. Die wird das heut’ mit Thraenen lesen. Und fragt man, wer ist Schuld daran Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Die Feldherrn, Staatschefs, Zeitungsknaben, Es fehlte damals nur ein atom, Ihn everyday im Munde haben. Haett er gesagt: I marry you madam. Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Even the sweethearts in the world(s) Dies ist nur unsre-erste Feier, Sie nennen sich jetzt: “Atom-girls.” Ich glaub die Sache wird noch teuer, Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. -

Opening Ceremony, 19 September 2010 Christian Colliex, IFSM
Opening Ceremony, 19 September 2010 Christian Colliex, IFSM President PartipantesPartipantes dodo IMCIMC 17,17, sejamsejam todostodos BemBem VindosVindos aoao RioRio DeDe JaneiroJaneiro IFSM International Federation of Societies for Microscopy Words about the Federation Life from 2006 to 2010 From IMC 16 in Sapporo to IMC 17 in Rio, and next? Prag ? Beijing? Istanbul ? Sapporo 2006 Rio 2010 Sydney ? General Assembly of IFSM selects Rio de Janeiro as host city for IMC 17 in 2010 Sapporo, closing ceremony GS and CC Sapporo 2006 IFSM presidents and executive board members Left to right : First row E. Zeitler, A. Maunsbach, G. Thomas,H. Hashimoto, A. Howie Second row : S. Iijima, G. Solorzano, B. Carter, G. Griffin, X, H. Lichte, D. Cockayne, P. Ingram, C. Colliex, W. Baumeister, K. Furuya. Selection and visit of the site (October 2006) Rehearsal, September 2009 From an IFSM summit meeting at Cusco (2007) preparing IMC 17… …to the official opening of IMC 17 to night at Rio de Janeiro 19 September 2010 Thanks and congratulations to the organizing committee Organizing Committee Chair Guillermo Solorzano Vice Chairs Wanderley de Souza (Life Science) Walter J. Botta (Materials Science) Daniel Ugarte (Instrumentation) Chair Local organizing committee Luiz Henrique de Almeida (UFRJ) Marcia Attias (UFRJ) Helene Barbosa (Fiocruz) Claudia Franca Barros (JBRJ) Ivani Bott (PUC‐Rio) Marilia Garcia Diniz (UERJ) Secretary Marcos Farina (UFRJ) Andréa Martiny (Inmetro) Rossana Melo (UFJF), secretary Vice Chairs André Luiz Pinto (CBPF) Elisa Saitovitch (CBPF) -

Rutherford and Radon
Rediscovery of the Elements Rutherford and Radon U transmutation of the elements. He was to be (1877-1956) he developed the "transformation U acknowledged as the "leading explorer of the theory" which showed radioactivity was a vast, infinitely complex universe within the nuclear property. From thorium Rutherford atom, a universe that he was the first to pene- observed a gaseous radioactive product, which trate."3 he called "emanation"-he had discovered the Ernest Rutherford, the son of a flax farmer in element radon.' New Zealand, gained his undergraduate train- The British method of developing explicit ing in his native country. In 1895 he moved to descriptive models to explain nature was per- England, where he attended Cambridge Univ- fect for the advance of nuclear chemistry at this ersity; he was entering the scientific scene in moment in history; Lord Kelvin (William Europe just as radioactivity was discovered in Thomson, 1824-1907) said he could not reason 1896 by Henri Becquerel (1852-1908) of Paris. "without making a visualizable picture" of the His first research involved hertzian (radio) phenomenon he wanted to describe. J. J. James L. Marshal, beta Eta 1971, and waves, but he then moved on to the study of Thomson of the Cavendish Laboratory at Virginia R. Marshall, Beta Eta 2003, uranium rays with J. J. Thomson (1856-1940) at Cambridge was using the idea of charged cor- Department of Chemistry, University of Cambridge. Rutherford showed that these ion- puscles to explain cathode rays, and he viewed North Texas, Denton,TX 76203-5070, izing rays consisted of two main types, which the atom as a dynamic, moving mixture of pos- he called alpha and beta "for simplicity."2 itive and negative charges. -

One Hundred Years of Chemical Warfare: Research
Bretislav Friedrich · Dieter Hoffmann Jürgen Renn · Florian Schmaltz · Martin Wolf Editors One Hundred Years of Chemical Warfare: Research, Deployment, Consequences One Hundred Years of Chemical Warfare: Research, Deployment, Consequences Bretislav Friedrich • Dieter Hoffmann Jürgen Renn • Florian Schmaltz Martin Wolf Editors One Hundred Years of Chemical Warfare: Research, Deployment, Consequences Editors Bretislav Friedrich Florian Schmaltz Fritz Haber Institute of the Max Planck Max Planck Institute for the History of Society Science Berlin Berlin Germany Germany Dieter Hoffmann Martin Wolf Max Planck Institute for the History of Fritz Haber Institute of the Max Planck Science Society Berlin Berlin Germany Germany Jürgen Renn Max Planck Institute for the History of Science Berlin Germany ISBN 978-3-319-51663-9 ISBN 978-3-319-51664-6 (eBook) DOI 10.1007/978-3-319-51664-6 Library of Congress Control Number: 2017941064 © The Editor(s) (if applicable) and The Author(s) 2017. This book is an open access publication. Open Access This book is licensed under the terms of the Creative Commons Attribution-NonCommercial 2.5 International License (http://creativecommons.org/licenses/by-nc/2.5/), which permits any noncom- mercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The images or other third party material in this book are included in the book's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the book's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.