Boxwood Blight Factsheet.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reconstructing the Basal Angiosperm Phylogeny: Evaluating Information Content of Mitochondrial Genes
55 (4) • November 2006: 837–856 Qiu & al. • Basal angiosperm phylogeny Reconstructing the basal angiosperm phylogeny: evaluating information content of mitochondrial genes Yin-Long Qiu1, Libo Li, Tory A. Hendry, Ruiqi Li, David W. Taylor, Michael J. Issa, Alexander J. Ronen, Mona L. Vekaria & Adam M. White 1Department of Ecology & Evolutionary Biology, The University Herbarium, University of Michigan, Ann Arbor, Michigan 48109-1048, U.S.A. [email protected] (author for correspondence). Three mitochondrial (atp1, matR, nad5), four chloroplast (atpB, matK, rbcL, rpoC2), and one nuclear (18S) genes from 162 seed plants, representing all major lineages of gymnosperms and angiosperms, were analyzed together in a supermatrix or in various partitions using likelihood and parsimony methods. The results show that Amborella + Nymphaeales together constitute the first diverging lineage of angiosperms, and that the topology of Amborella alone being sister to all other angiosperms likely represents a local long branch attrac- tion artifact. The monophyly of magnoliids, as well as sister relationships between Magnoliales and Laurales, and between Canellales and Piperales, are all strongly supported. The sister relationship to eudicots of Ceratophyllum is not strongly supported by this study; instead a placement of the genus with Chloranthaceae receives moderate support in the mitochondrial gene analyses. Relationships among magnoliids, monocots, and eudicots remain unresolved. Direct comparisons of analytic results from several data partitions with or without RNA editing sites show that in multigene analyses, RNA editing has no effect on well supported rela- tionships, but minor effect on weakly supported ones. Finally, comparisons of results from separate analyses of mitochondrial and chloroplast genes demonstrate that mitochondrial genes, with overall slower rates of sub- stitution than chloroplast genes, are informative phylogenetic markers, and are particularly suitable for resolv- ing deep relationships. -
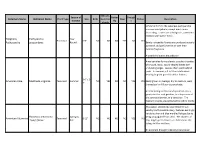
Common Name Botanical Name Alleghany
Attracts Season of Butter Drough Common Name Botanical Name Plant Type Size Birds Hummin Deer Native Description Interest fly t g birds Similar in form to the Japanese pachysandra one sees everywhere, except much more interesting. Leaves are a dull green, sometimes mottled with lighter flecks. Alleghany Pachysandra Year Perennial 6-8" NO NO NO YES NO YES Pachysandra procumbens Round Barely noticeable flowers are produced as early as March and perfume the air with their delicate fragrance. A wonderful native groundcover. American aloe forms a lovely succulent rosette of smooth, waxy, sword-shaped leaves with undulating edges. Leaves often sport reddish spots. In summer, a 3 to 5 foot stalk arises bearing fragrant greenish-white flowers. 3-6' x 2- American Aloe Manfreda virginica Perennial Summer NO YES NO NO YES YES Easily grown in average, dry to medium, well- 3' drained soil in full sun to part shade. An interesting architectural specimen, it is a good plant for rock gardens, in a dry corner of the perennial border, or a container. The fragrant blooms are pollinated by sphinx moths. This native, selected by Dale Hendrick's at nearby North Creek Nursery, features excitingly variable silver and blue marbled foliage due to Heuchera americana Spring to being propagated from seed. The clusters of American Alumroot Perennial 8-12" NO NO NO NO YES NO 'Dale's Strain' Fall tiny, bright green flowers are held above the foliage in May and June. An excellent drought tolerant groundcover. Viburnum trilobum is a native deciduous shrub to the northeastern and northwestern United States. -

Boxwood Blight: a New Ornamental Disease Threat
DIVISION OF AGRICULTURE Agriculture and Natural Resources RESEARCH & EXTENSION University of Arkansas System FSA7577 Boxwood Blight: A New Ornamental Disease Threat Keiddy E. Importance (Figure 2). Although the plant attempts Urrea-Morawicki to regrow, repeated infections and Boxwood blight caused by two defoliation weaken it and can lead to Instructor/Diagnostician - related fungi, Calonectria pseud- Plant Health Clinic plant death. Dark brown to black stem onaviculata, (previously known as streak or cankers are also symptoms of Cylindrocladium pseudonaviculatum infection (Figure 3). These lesions can Sherrie E. Smith or. Cylindrocladium buxicola) and Plant Pathologist/Instructor be found occurring anywhere from the Calonectria henricotiae (previously Plant Health Clinic soil line to the twig tips. Under high known as Cylindrocladium buxicola) humidity conditions, masses of white is an important disease of Boxwood fruiting bodies are produced on the (Boxus). Boxwood blight was first undersurface of infected leaves (Figure described in the United Kingdom in the 4), containing the distinctive spores of mid-1990s and in New Zealand in 2002. the fungi (Figure 5). These white struc- Since these initial reports, the disease tures are readily visible with a hand has become widespread throughout the lens and are useful in distinguishing United Kingdom, New Zealand, Europe boxwood blight from another boxwood and Asia. In the United States, Boxwood disease, Volutella blight. The Volutella blight was reported for the first time blight fungus (Volutella buxi) produces in 2011 in North Carolina and salmon colored fruiting bodies on leaf Connecticut. By 2018, twenty-five states undersurfaces. Symptoms of Volutella had confirmed the disease, and it was blight and Phytophthora root rot are first identified in Arkansas in Pulaski sometimes confused with boxwood county in 2019. -

Native Vascular Plants
!Yt q12'5 3. /3<L....:::5_____ ,--- _____ Y)Q.'f MUSEUM BULLETIN NO.4 -------------- Copy I NATIVE VASCULAR PLANTS Endangered, Threatened, Or Otherwise In Jeopardy In South Carolina By Douglas A. Rayner, Chairman And Other Members Of The South Carolina Advisory Committee On Endangered, Threatened And Rare Plants SOUTH CAROLINA MUSEUM COMMISSION S. C. STATE LIR7~'· '?Y rAPR 1 1 1995 STATE DOCU~ 41 ;::,·. l s NATIVE VASCULAR PLANTS ENDANGERED, THREATENED, OR OTHERWISE IN JEOPARDY IN SOUTH CAROLINA by Douglas A. Rayner, Chairman and other members of the South Carolina Advisory Committee on Endangered, Threatened, and Rare Plants March, 1979 Current membership of the S. C. Committee on Endangered, Threatened, and Rare Plants Subcommittee on Criteria: Ross C. Clark, Chairman (1977); Erskine College (taxonomy and ecology) Steven M. Jones, Clemson University (forest ecology) Richard D. Porcher, The Citadel (taxonomy) Douglas A. Rayner, S.C. Wildlife Department (taxonomy and ecology) Subcommittee on Listings: C. Leland Rodgers, Chairman (1977 listings); Furman University (taxonomy and ecology) Wade T. Batson, University of South Carolina, Columbia (taxonomy and ecology) Ross C. Clark, Erskine College (taxonomy and ecology) John E. Fairey, III, Clemson University (taxonomy) Joseph N. Pinson, Jr., University of South Carolina, Coastal Carolina College (taxonomy) Robert W. Powell, Jr., Converse College (taxonomy) Douglas A Rayner, Chairman (1979 listings) S. C. Wildlife Department (taxonomy and ecology) INTRODUCTION South Carolina's first list of rare vascular plants was produced as part of the 1976 S.C. En dangered Species Symposium by the S. C. Advisory Committee on Endangered, Threatened and Rare Plants, 1977. The Symposium was a joint effort of The Citadel's Department of Biology and the S. -

Fv{Âçä~|ÄÄ Géãçá{|Ñ CHESTER COUNTY PENNSYLVANIA
fv{âçÄ~|ÄÄ gÉãÇá{|Ñ CHESTER COUNTY PENNSYLVANIA cxÇÇáçÄätÇ|t fàtàx YÄÉãxÜ `ÉâÇàt|Ç _tâÜxÄ NATIVE PLANT LIST A RESIDENT’S GUIDE 2017 fv{âçÄ~|ÄÄ gÉãÇá{|Ñ CHESTER COUNTY NATIVE PLANT LIST A Resident’s Guide Contents INTRODUCTION .................................................................................................... 1 So what exactly is a Native Plant? ................................................................. 1 Go native with these 6 basics: ........................................................................ 2 In Summary ....................................................................................................... 4 NATIVE PLANT LIST OVERVIEW ............................................................................. 5 TREES ................................................................................................................. 6 EVERGREEN ................................................................................................... 6 DECIDUOUS ................................................................................................... 6 FLOWERING ................................................................................................... 7 SHRUBS .............................................................................................................. 8 EVERGREEN ................................................................................................... 8 DECIDUOUS .................................................................................................. -

Marcia Winchester, Cherokee County Master Gardener AUGUST
For the Cherokee County Master Gardeners Volume XXIV, Issue 5 Aug/Sept 2017 What’s Happening Editor’s Corner By Marcia Winchester, Cherokee County Master Gardener AUGUST Aug 3 - Demo Garden workday Fall is very important to a lot of our pollinators. Is your garden ready 9:30-12 for them? Fall is the time when our pollinators get ready for winter. Aug 8 - Papa’s Pantry workday Many have completed their life cycles to the adult stage. A lot of native bees have already returned to their ground nests. Tilling gardens can Aug 15 - Monthly Meeting destroy their dwellings and disrupt their life cycles. Leaving piles of Aug 15 - Demo Garden workday sticks and fallen leaves will give many pollinators a safe place to 9:30-12 overwinter. Aug 24 - Papa’s Pantry workday Fall-blooming plants like goldenrods (Solidago spp.) and asters Aug 26 - Lecture on Fall Vegetable Gardening, Hickory Flat (Symphyotrichum spp. and Eurybia spp.) provide nectar for a large Library @ 10am number of pollinators including butterflies and bees. Some butterflies, such as the monarch, need to bulk up on nectar to successfully migrate SEPTEMBER to warmer climates where they spend the winter. Hummingbirds have to double their weight to migrate south. Be sure to add nectar plants Sep 7 - Demo Garden workday like ironweed (Vernonia spp.), dwarf blazing star (Liatris pilosa), Texas 9:30-12 hibiscus (Hibiscus coccineus), green-headed coneflower (Rudbeckia Sep 8 - Fall Plant Sale set up laciniata), turtlehead (Chelone glabra), and salvias such as Salvia Sep 9 - Fall Plant Sale, 9-12 guaranitica ‘Black and Blue’ to your gardens. -

Quarantine Host Range and Natural History of Gadirtha Fusca, a Potential Biological Control Agent of Chinese Tallowtree (Triadica Sebifera) in North America
DOI: 10.1111/eea.12737 Quarantine host range and natural history of Gadirtha fusca, a potential biological control agent of Chinese tallowtree (Triadica sebifera) in North America Gregory S. Wheeler1* , Emily Jones1, Kirsten Dyer1, Nick Silverson1 & Susan A. Wright2 1USDA/ARS Invasive Plant Research Laboratory, 3225 College Ave., Ft Lauderdale, FL 33314, USA, and 2USDA/ARS Invasive Plant Research Laboratory, Gainesville, FL 32608, USA Accepted: 23 August 2018 Key words: biocontrol, classical biological control, weed control, Euphorbiaceae, defoliating caterpillar, host range tests, invasive weeds, Sapium, Lepidoptera, Nolidae, integrated pest management, IPM Abstract Classical biological control can provide an ecologically sound, cost-effective, and sustainable manage- ment solution to protect diverse habitats. These natural and managed ecosystems are being invaded and transformed by invasive species. Chinese tallowtree, Triadica sebifera (L.) Small (Euphorbiaceae), is one of the most damaging invasive weeds in the southeastern USA, impacting wetlands, forests, and natural areas. A defoliating moth, Gadirtha fusca Pogue (Lepidoptera: Nolidae), was discovered feeding on Chinese tallowtree leaves in the weed’s native range and has been tested for its suitability as a biological control agent. Natural history studies of G. fusca indicated that the neonates have five instars and require 15.4 days to reach pupation. Complete development from egg hatch to adult emergence required 25.8 days. No differences were found between males and females in terms of life history and nutritional indices measured. Testing of the host range of G. fusca larvae was conducted with no-choice, dual-choice, and multigeneration tests and the results indicated that this species has a very narrow host range. -

High Line Plant List Stay Connected @Highlinenyc
BROUGHT TO YOU BY HIGH LINE PLANT LIST STAY CONNECTED @HIGHLINENYC Trees & Shrubs Acer triflorum three-flowered maple Indigofera amblyantha pink-flowered indigo Aesculus parviflora bottlebrush buckeye Indigofera heterantha Himalayan indigo Amelanchier arborea common serviceberry Juniperus virginiana ‘Corcorcor’ Emerald Sentinel® eastern red cedar Amelanchier laevis Allegheny serviceberry Emerald Sentinel ™ Amorpha canescens leadplant Lespedeza thunbergii ‘Gibraltar’ Gibraltar bushclover Amorpha fruticosa desert false indigo Magnolia macrophylla bigleaf magnolia Aronia melanocarpa ‘Viking’ Viking black chokeberry Magnolia tripetala umbrella tree Betula nigra river birch Magnolia virginiana var. australis Green Shadow sweetbay magnolia Betula populifolia grey birch ‘Green Shadow’ Betula populifolia ‘Whitespire’ Whitespire grey birch Mahonia x media ‘Winter Sun’ Winter Sun mahonia Callicarpa dichotoma beautyberry Malus domestica ‘Golden Russet’ Golden Russet apple Calycanthus floridus sweetshrub Malus floribunda crabapple Calycanthus floridus ‘Michael Lindsey’ Michael Lindsey sweetshrub Nyssa sylvatica black gum Carpinus betulus ‘Fastigiata’ upright European hornbeam Nyssa sylvatica ‘Wildfire’ Wildfire black gum Carpinus caroliniana American hornbeam Philadelphus ‘Natchez’ Natchez sweet mock orange Cercis canadensis eastern redbud Populus tremuloides quaking aspen Cercis canadensis ‘Ace of Hearts’ Ace of Hearts redbud Prunus virginiana chokecherry Cercis canadensis ‘Appalachian Red’ Appalachian Red redbud Ptelea trifoliata hoptree Cercis -

Wildflowers and Ferns Along the Acton Arboretum Wildflower Trail and in Other Gardens FERNS (Including Those Occurring Naturally
Wildflowers and Ferns Along the Acton Arboretum Wildflower Trail and In Other Gardens Updated to June 9, 2018 by Bruce Carley FERNS (including those occurring naturally along the trail and both boardwalks) Royal fern (Osmunda regalis): occasional along south boardwalk, at edge of hosta garden, and elsewhere at Arboretum Cinnamon fern (Osmunda cinnamomea): naturally occurring in quantity along south boardwalk Interrupted fern (Osmunda claytoniana): naturally occurring in quantity along south boardwalk Maidenhair fern (Adiantum pedatum): several healthy clumps along boardwalk and trail, a few in other Arboretum gardens Common polypody (Polypodium virginianum): 1 small clump near north boardwalk Hayscented fern (Dennstaedtia punctilobula): aggressive species; naturally occurring along north boardwalk Bracken fern (Pteridium aquilinum): occasional along wildflower trail; common elsewhere at Arboretum Broad beech fern (Phegopteris hexagonoptera): up to a few near north boardwalk; also in rhododendron and hosta gardens New York fern (Thelypteris noveboracensis): naturally occurring and abundant along wildflower trail * Ostrich fern (Matteuccia pensylvanica): well-established along many parts of wildflower trail; fiddleheads edible Sensitive fern (Onoclea sensibilis): naturally occurring and abundant along south boardwalk Lady fern (Athyrium filix-foemina): moderately present along wildflower trail and south boardwalk Common woodfern (Dryopteris spinulosa): 1 patch of 4 plants along south boardwalk; occasional elsewhere at Arboretum Marginal -

Green Factor Plant List 2010
Revised December 2010 Seattle Green Factor Plant List Notes: ● All plants on this list are drought-tolerant once they are established unless comments indicate otherwise. ● Seattle Department of Transportations Right-of Way Improvement Manual establishes height limits for non-street-tree plantings in rights-of-way. Maximum plant height within 30 feet of an intersection (as measured from the corner of the curb) is 24 inches. Elsewhere in the right-of-way, plantings are allowed to be 30 inches tall. ● "Bioretention Zone" describes where plants can appropriately be used in bioretention systems such as swales and rain gardens. Zone 1 is the designation for plants that can be used in the flat bottoms of bioretention facilities: 1A refers to species that prefer soil saturation or shallow inundation for long durations, while Zone 1B refers to plants that can alternate between dry ands short-term saturated conditions. Zone 2 is the designation for plants best used at the well-drained slopes of bioretention facilities. All other species are appropriate for planting at the tops of bioretention areas. GROUNDCOVERS Scientific Name Common Name Evergreen Shade Sun Native up to 24" 2-3' ht Bioretention Zone Notes Arctostaphylos uva-ursi kinnikinnick ●●●● Asarum caudatum wild ginger ●● ● Calluna , in variety heather ●●● Ceratostigma plumbaginoides hardy plumbago ●●● ● Daboecia cantabrica Irish heath ●●● Erica , in variety heath ●●● Erigeron karvinskianus Latin American fleabane ●●● ● Euonymous fortunei 'Colorata' wintercreeper euonymous ●●● ● Festuca -

Ground Covers for the Chicago Area
GROUND COVERS FOR THE CHICAGO AREA A ground cover plant is any low-growing or trailing plant used in recommendations. When using chemicals, follow all label the landscape to cover exposed areas of soil. A wide variety of directions carefully. plants may be used as groundcover, including perennial herbaceous plants, deciduous and or evergreen woody plants, 3: Evaluate the soil. Determine whether the soil is suitable for and vary in height from 1 inch to 3-4 feet. This fact sheet root growth or if it must be improved. In the Chicago area most focuses on herbaceous ground covers. Please refer to Woody garden soils are heavy clay and are slow to drain. This problem Ground Covers for the Chicago Area for information on non- can be corrected with the addition of proper soil amendments. If herbaceous ground covers. using plants that have pH preferences, a soil test should be taken to check the fertility and pH level of the soil prior to planting. At one time, turf grass was the most common ground cover, but Refer to the Garden Soils Fact Sheet for specific guidelines many gardeners now find lower maintenance plants more regarding soil amendments and testing. desirable. There are several different reasons for choosing other plants over turf grasses. Many ground covers require less 4: Add soil amendments, if necessary. Be careful when adding maintenance than turf. This is especially important when dealing amendments beneath established trees and shrubs. No more with small areas, corners or edges of the yard where mowing is than 2-3 inches should be added at any time to avoid compacting difficult. -

Gardening with New York City Native Plants
Gardening with New York City Native Plants Smooth Rose Flowering dogwood City of New York Parks & Recreation Michael R. Bloomberg, Mayor Adrian Benepe, Commissioner Bill Tai, Director, Natural Resources Group www.nyc.gov/parks Solomon’s seal Jack in the pulpit Violet Wild ginger Butterfly weed Columbine What is a native plant? Why go native in the garden? A native plant is one that naturally occurs in a region without having Sense of place: Why do yards and window boxes across the been introduced from elsewhere by people. New York City natives country hold the same impatiens, begonias and mums? Most of include mosses, ferns, grasses, sedges and rushes, wildflowers, trees, America’s favorite garden plants hail from places like Europe and Asia. shrubs, and vines. The New York area has its own regional flavor and distinct assemblage of native plants. We should seek out alternatives to hardware stores, Over thousands of years, native plants have adapted to the climate, corner delis and other outlets that offer “one size fits all”. The NYC soils, and environmental conditions of our area. They have developed Greenmarkets, with their emphasis on locally grown greenery, can the ability to thrive in our humid summers and freezing winters and to help you cultivate a sense of home by sowing local seeds. entice local insects, birds, and other animals to pollinate their flowers and disperse their fruits. Native plants are responsible for clean air, Ease of care: When installed in the appropriate habitat, native pure water, soil stability, flood abatement, and wild animal habitat. plants require less maintenance than the exotic alternatives.