VAST−Class−A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Thioredoxin Trx-1 Regulates the Major Oxidative Stress Response Transcription Factor, Skn-1, in Caenorhabditis Elegans
The Texas Medical Center Library DigitalCommons@TMC The University of Texas MD Anderson Cancer Center UTHealth Graduate School of The University of Texas MD Anderson Cancer Biomedical Sciences Dissertations and Theses Center UTHealth Graduate School of (Open Access) Biomedical Sciences 5-2016 THE THIOREDOXIN TRX-1 REGULATES THE MAJOR OXIDATIVE STRESS RESPONSE TRANSCRIPTION FACTOR, SKN-1, IN CAENORHABDITIS ELEGANS Katie C. McCallum Follow this and additional works at: https://digitalcommons.library.tmc.edu/utgsbs_dissertations Part of the Cellular and Molecular Physiology Commons, Medicine and Health Sciences Commons, Molecular Genetics Commons, and the Organismal Biological Physiology Commons Recommended Citation McCallum, Katie C., "THE THIOREDOXIN TRX-1 REGULATES THE MAJOR OXIDATIVE STRESS RESPONSE TRANSCRIPTION FACTOR, SKN-1, IN CAENORHABDITIS ELEGANS" (2016). The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access). 655. https://digitalcommons.library.tmc.edu/utgsbs_dissertations/655 This Dissertation (PhD) is brought to you for free and open access by the The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences at DigitalCommons@TMC. It has been accepted for inclusion in The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access) by an authorized administrator of DigitalCommons@TMC. For more information, please contact [email protected]. THE THIOREDOXIN TRX-1 REGULATES THE MAJOR OXIDATIVE STRESS RESPONSE TRANSCRIPTION FACTOR, SKN-1, IN CAENORHABDITIS ELEGANS A DISSERTATION Presented to the Faculty of The University of Texas Health Science Center at Houston and The University of Texas MD Anderson Cancer Center Graduate School of Biomedical Sciences in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY by Katie Carol McCallum, B.S. -

Molecular Mechanisms Involved Involved in the Interaction Effects of HCV and Ethanol on Liver Cirrhosis
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2010 Molecular Mechanisms Involved Involved in the Interaction Effects of HCV and Ethanol on Liver Cirrhosis Ryan Fassnacht Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Physiology Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/2246 This Thesis is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. Ryan C. Fassnacht 2010 All Rights Reserved Molecular Mechanisms Involved in the Interaction Effects of HCV and Ethanol on Liver Cirrhosis A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science at Virginia Commonwealth University. by Ryan Christopher Fassnacht, B.S. Hampden Sydney University, 2005 M.S. Virginia Commonwealth University, 2010 Director: Valeria Mas, Ph.D., Associate Professor of Surgery and Pathology Division of Transplant Department of Surgery Virginia Commonwealth University Richmond, Virginia July 9, 2010 Acknowledgement The Author wishes to thank his family and close friends for their support. He would also like to thank the members of the molecular transplant team for their help and advice. This project would not have been possible with out the help of Dr. Valeria Mas and her endearing -

Muscarinic Acetylcholine Type 1 Receptor Activity Constrains Neurite Outgrowth by Inhibiting Microtubule Polymerization and Mito
fnins-12-00402 June 26, 2018 Time: 12:46 # 1 ORIGINAL RESEARCH published: 26 June 2018 doi: 10.3389/fnins.2018.00402 Muscarinic Acetylcholine Type 1 Receptor Activity Constrains Neurite Outgrowth by Inhibiting Microtubule Polymerization and Mitochondrial Trafficking in Adult Sensory Neurons Mohammad G. Sabbir1*, Nigel A. Calcutt2 and Paul Fernyhough1,3 1 Division of Neurodegenerative Disorders, St. Boniface Hospital Research Centre, Winnipeg, MB, Canada, 2 Department of Pathology, University of California, San Diego, San Diego, CA, United States, 3 Department of Pharmacology and Therapeutics, University of Manitoba, Winnipeg, MB, Canada The muscarinic acetylcholine type 1 receptor (M1R) is a metabotropic G protein-coupled Edited by: receptor. Knockout of M1R or exposure to selective or specific receptor antagonists Roberto Di Maio, elevates neurite outgrowth in adult sensory neurons and is therapeutic in diverse University of Pittsburgh, United States models of peripheral neuropathy. We tested the hypothesis that endogenous M1R Reviewed by: activation constrained neurite outgrowth via a negative impact on the cytoskeleton Roland Brandt, University of Osnabrück, Germany and subsequent mitochondrial trafficking. We overexpressed M1R in primary cultures Rick Dobrowsky, of adult rat sensory neurons and cell lines and studied the physiological and The University of Kansas, United States molecular consequences related to regulation of cytoskeletal/mitochondrial dynamics *Correspondence: and neurite outgrowth. In adult primary neurons, overexpression of M1R caused Mohammad G. Sabbir disruption of the tubulin, but not actin, cytoskeleton and significantly reduced neurite [email protected] outgrowth. Over-expression of a M1R-DREADD mutant comparatively increased neurite Specialty section: outgrowth suggesting that acetylcholine released from cultured neurons interacts This article was submitted to with M1R to suppress neurite outgrowth. -

The Unique Cysteine Knot Regulates the Pleotropic Hormone Leptin
The Unique Cysteine Knot Regulates the Pleotropic Hormone Leptin Ellinor Haglund1, Joanna I. Sułkowska1, Zhao He2, Gen-Sheng Feng2, Patricia A. Jennings1*, Jose´ N. Onuchic3* 1 Department of Chemistry and Biochemistry and Center for theoretical Biological Physics (CTBP), University of California San Diego, La Jolla, California, United States of America, 2 Department of Pathology; School of Medicine and Molecular Biology Section, Division of Biological Sciences, University of California San Diego, La Jolla, California, United States of America, 3 Center for Theoretical Biological physics and Department of Physics and Astronomy, Chemistry, and Biochemistry and Cell Biology, Rice University, Houston, Texas, United States of America Abstract Leptin plays a key role in regulating energy intake/expenditure, metabolism and hypertension. It folds into a four-helix bundle that binds to the extracellular receptor to initiate signaling. Our work on leptin revealed a hidden complexity in the formation of a previously un-described, cysteine-knotted topology in leptin. We hypothesized that this unique topology could offer new mechanisms in regulating the protein activity. A combination of in silico simulation and in vitro experiments was used to probe the role of the knotted topology introduced by the disulphide-bridge on leptin folding and function. Our results surprisingly show that the free energy landscape is conserved between knotted and unknotted protein, however the additional complexity added by the knot formation is structurally important. Native state analyses led to the discovery that the disulphide-bond plays an important role in receptor binding and thus mediate biological activity by local motions on distal receptor-binding sites, far removed from the disulphide-bridge. -

Structure of the Thioredoxin-Fold Domain of Human Phosducin-Like
protein structure communications Acta Crystallographica Section F Structural Biology Structure of the thioredoxin-fold domain of human and Crystallization phosducin-like protein 2 Communications ISSN 1744-3091 Xiaochu Lou,a Rui Bao,a Human phosducin-like protein 2 (hPDCL2) has been identified as belonging to Cong-Zhao Zhoub and subgroup II of the phosducin (Pdc) family. The members of this family share an Yuxing Chena,b* N-terminal helix domain and a C-terminal thioredoxin-fold (Trx-fold) domain. The X-ray crystal structure of the Trx-fold domain of hPDCL2 was solved at ˚ aInstitute of Protein Research, Tongji University, 2.70 A resolution and resembled the Trx-fold domain of rat phosducin. Shanghai 200092, People’s Republic of China, Comparative structural analysis revealed the structural basis of their putative and bHefei National Laboratory for Physical functional divergence. Sciences at Microscale and School of Life Sciences, University of Science and Technology of China, Hefei, Anhui 230027, People’s Republic of China Correspondence e-mail: [email protected] 1. Introduction The thioredoxin-fold (Trx-fold) protein-structure classification (SCOP; Received 21 October 2008 http://scop.mrc-lmb.cam.ac.uk; Murzin et al., 1995) was characterized Accepted 11 November 2008 based on the structure of Escherichia coli Trx1 (Holmgren et al., 1975). The classic Trx fold consists of a single domain with a central PDB Reference: thioredoxin-fold domain of five-stranded mixed -sheet flanked by two -helices on each side. human phosducin-like protein 2, 3evi, r3evisf. The secondary-structure elements are arranged in the order - , with 4 antiparallel to the other strands. -

Redundancy in Ribonucleotide Excision Repair: Competition, Compensation, and Cooperation
DNA Repair 29 (2015) 74–82 Contents lists available at ScienceDirect DNA Repair j ournal homepage: www.elsevier.com/locate/dnarepair Redundancy in ribonucleotide excision repair: Competition, compensation, and cooperation ∗ Alexandra Vaisman, Roger Woodgate Laboratory of Genomic Integrity, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892-3371, USA a r t i c l e i n f o a b s t r a c t Article history: The survival of all living organisms is determined by their ability to reproduce, which in turn depends Received 1 November 2014 on accurate duplication of chromosomal DNA. In order to ensure the integrity of genome duplication, Received in revised form 7 February 2015 DNA polymerases are equipped with stringent mechanisms by which they select and insert correctly Accepted 9 February 2015 paired nucleotides with a deoxyribose sugar ring. However, this process is never 100% accurate. To fix Available online 16 February 2015 occasional mistakes, cells have evolved highly sophisticated and often redundant mechanisms. A good example is mismatch repair (MMR), which corrects the majority of mispaired bases and which has been Keywords: extensively studied for many years. On the contrary, pathways leading to the replacement of nucleotides Ribonucleotide excision repair with an incorrect sugar that is embedded in chromosomal DNA have only recently attracted significant Nucleotide excision repair attention. This review describes progress made during the last few years in understanding such path- Mismatch repair Ribonuclease H ways in both prokaryotes and eukaryotes. Genetic studies in Escherichia coli and Saccharomyces cerevisiae Flap endonuclease demonstrated that MMR has the capacity to replace errant ribonucleotides, but only when the base is DNA polymerase I mispaired. -

Polymerase Ribozyme with Promoter Recognition
In vitro Evolution of a Processive Clamping RNA Polymerase Ribozyme with Promoter Recognition by Razvan Cojocaru BSc, Simon Fraser University, 2014 Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in the Department of Molecular Biology and Biochemistry Faculty of Science © Razvan Cojocaru 2021 SIMON FRASER UNIVERSITY Summer 2021 Copyright in this work is held by the author. Please ensure that any reproduction or re-use is done in accordance with the relevant national copyright legislation. Declaration of Committee Name: Razvan Cojocaru Degree: Doctor of Philosophy Title: In vitro Evolution of a Processive Clamping RNA Polymerase Ribozyme with Promoter Recognition Committee: Chair: Lisa Craig Professor, Molecular Biology and Biochemistry Peter Unrau Supervisor Professor, Molecular Biology and Biochemistry Dipankar Sen Committee Member Professor, Molecular Biology and Biochemistry Michel Leroux Committee Member Professor, Molecular Biology and Biochemistry Mani Larijani Internal Examiner Associate Professor, Molecular Biology and Biochemistry Gerald Joyce External Examiner Professor, Jack H. Skirball Center for Chemical Biology and Proteomics Salk Institute for Biological Studies Date Defended/Approved: August 12, 2021 ii Abstract The RNA World hypothesis proposes that the early evolution of life began with RNAs that can serve both as carriers of genetic information and as catalysts. Later in evolution, these functions were gradually replaced by DNA and enzymatic proteins in cellular biology. I start by reviewing the naturally occurring catalytic RNAs, ribozymes, as they play many important roles in biology today. These ribozymes are central to protein synthesis and the regulation of gene expression, creating a landscape that strongly supports an early RNA World. -

Supporting Information
Supporting Information Figure S1. The functionality of the tagged Arp6 and Swr1 was confirmed by monitoring cell growth and sensitivity to hydeoxyurea (HU). Five-fold serial dilutions of each strain were plated on YPD with or without 50 mM HU and incubated at 30°C or 37°C for 3 days. Figure S2. Localization of Arp6 and Swr1 on chromosome 3. The binding of Arp6-FLAG (top), Swr1-FLAG (middle), and Arp6-FLAG in swr1 cells (bottom) are compared. The position of Tel 3L, Tel 3R, CEN3, and the RP gene are shown under the panels. Figure S3. Localization of Arp6 and Swr1 on chromosome 4. The binding of Arp6-FLAG (top), Swr1-FLAG (middle), and Arp6-FLAG in swr1 cells (bottom) in the whole chromosome region are compared. The position of Tel 4L, Tel 4R, CEN4, SWR1, and RP genes are shown under the panels. Figure S4. Localization of Arp6 and Swr1 on the region including the SWR1 gene of chromosome 4. The binding of Arp6- FLAG (top), Swr1-FLAG (middle), and Arp6-FLAG in swr1 cells (bottom) are compared. The position and orientation of the SWR1 gene is shown. Figure S5. Localization of Arp6 and Swr1 on chromosome 5. The binding of Arp6-FLAG (top), Swr1-FLAG (middle), and Arp6-FLAG in swr1 cells (bottom) are compared. The position of Tel 5L, Tel 5R, CEN5, and the RP genes are shown under the panels. Figure S6. Preferential localization of Arp6 and Swr1 in the 5′ end of genes. Vertical bars represent the binding ratio of proteins in each locus. -
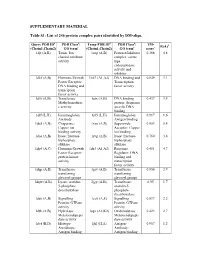
List of 246 Protein Complex Pairs Identified by DM-Align
SUPPLEMENTARY MATERIAL Table S1: List of 246 protein complex pairs identified by DM-align. Query PDB IDa PDB Classb: Temp PDB IDd PDB Classb: TM- R(Å)f (Chain1,Chain2) GO termc (Chain1,Chain2) GO termc scoree 1djt (A,B) Toxin: Ion 1aap (A,B) Protease/Inhibitor 0.368 4.8 channel inhibitor complex: serine activity type endopeptidase activity and inhibitor 1dkf (A,B) Hormone/Growth 1xb7 (A1,A2) DNA binding and 0.849 3.1 Factor Receptor: Transcription DNA binding and factor activity transciption factor activity 1dl5 (A,B) Transferase: 1utx (A,B) DNA binding 0.437 3.9 Methyltransferas protein: Sequence e activity specific DNA binding 1dlf (L,H) Immunoglobin: 1j05 (L,H) Immunoglobin: 0.917 1.6 Antibody Antigen binding 1do5 (A,B) Chaperone: 1xso (A,B) Superoxide 0.953 0.9 Copper ion Acceptor: Copper binding activity ion binding 1dos (A,B) lyase: fructose- 1rvg (A,B) lyase: fructose- 0.760 3.6 biphosphate biphosphate aldolase aldolase 1dp4 (A,C) Hormone/Growth 1dz3 (A1,A2) Response 0.481 4.7 Factor Receptor: Regulator: DNA protein kinase binding and activity transcription factor activity 1dqp (A,B) Transferase: 1grv (A,B) Transferase: 0.856 2.9 transferring transferring glycosyl groups glycosyl groups 1dqw (A,B) Lyase: orotidin- 2jgy (A,B) Transferase: 0.95 1.7 5-phosphate orotidin-5- decarboxylase phosphate decarboxylase 1ds6 (A,B) Signalling 1cc0 (A,E) Signalling 0.897 2.2 Protein: GTPase Protein: GTPase activity activity 1dth (A,B) Hydrolase: 1sqv (A2,K2) Oxidoredutase: 0.423 2.7 Metaloendopepti Metaloendopepti dase activity dase activity 1dvf -

Yeast Genome Gazetteer P35-65
gazetteer Metabolism 35 tRNA modification mitochondrial transport amino-acid metabolism other tRNA-transcription activities vesicular transport (Golgi network, etc.) nitrogen and sulphur metabolism mRNA synthesis peroxisomal transport nucleotide metabolism mRNA processing (splicing) vacuolar transport phosphate metabolism mRNA processing (5’-end, 3’-end processing extracellular transport carbohydrate metabolism and mRNA degradation) cellular import lipid, fatty-acid and sterol metabolism other mRNA-transcription activities other intracellular-transport activities biosynthesis of vitamins, cofactors and RNA transport prosthetic groups other transcription activities Cellular organization and biogenesis 54 ionic homeostasis organization and biogenesis of cell wall and Protein synthesis 48 plasma membrane Energy 40 ribosomal proteins organization and biogenesis of glycolysis translation (initiation,elongation and cytoskeleton gluconeogenesis termination) organization and biogenesis of endoplasmic pentose-phosphate pathway translational control reticulum and Golgi tricarboxylic-acid pathway tRNA synthetases organization and biogenesis of chromosome respiration other protein-synthesis activities structure fermentation mitochondrial organization and biogenesis metabolism of energy reserves (glycogen Protein destination 49 peroxisomal organization and biogenesis and trehalose) protein folding and stabilization endosomal organization and biogenesis other energy-generation activities protein targeting, sorting and translocation vacuolar and lysosomal -

Evidence for Conformational Change-Induced Hydrolysis of Β-Tubulin-GTP
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.08.288019; this version posted September 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Evidence for conformational change-induced hydrolysis of β-tubulin-GTP Mohammadjavad Paydar1 and Benjamin H. Kwok1† 1 Institute for Research in Immunology and Cancer (IRIC), Département de médecine, Université de Montréal, P.O. Box 6128, Station Centre-Ville, Montréal, QC H3C 3J7, Canada. † Corresponding author: B. H. Kwok: [email protected] Keywords: Kinesin, Kif2A, MCAK, motor protein, microtubules, tubulin, GTP hydrolysis Abbreviations NM: Neck+Motor MD: Motor domain MT: Microtubule Running title: Tubulin GTP hydrolysis 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.09.08.288019; this version posted September 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. ABSTRACT Microtubules, protein polymers of α/β-tubulin dimers, form the structural framework for many essential cellular processes including cell shape formation, intracellular transport, and segregation of chromosomes during cell division. It is known that tubulin-GTP hydrolysis is closely associated with microtubule polymerization dynamics. However, the precise roles of GTP hydrolysis in tubulin polymerization and microtubule depolymerization, and how it is initiated are still not clearly defined. We report here that tubulin-GTP hydrolysis can be triggered by conformational change induced by the depolymerizing kinesin-13 proteins or by the stabilizing chemical agent paclitaxel. We provide biochemical evidence that conformational change precedes tubulin-GTP hydrolysis, confirming this process is mechanically driven and structurally directional. -

Chemical Synthesis and NMR Solution Structure of Conotoxin GXIA from Conus Geographus
marine drugs Article Chemical Synthesis and NMR Solution Structure of Conotoxin GXIA from Conus geographus David A. Armstrong 1, Ai-Hua Jin 2, Nayara Braga Emidio 2 , Richard J. Lewis 2 , Paul F. Alewood 2 and K. Johan Rosengren 1,* 1 School of Biomedical Sciences, Faculty of Medicine, The University of Queensland, Brisbane, QLD 4072, Australia; [email protected] 2 Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD 4072, Australia; [email protected] (A.-H.J.); [email protected] (N.B.E.); [email protected] (R.J.L.); [email protected] (P.F.A.) * Correspondence: [email protected] Abstract: Conotoxins are disulfide-rich peptides found in the venom of cone snails. Due to their exquisite potency and high selectivity for a wide range of voltage and ligand gated ion channels they are attractive drug leads in neuropharmacology. Recently, cone snails were found to have the capability to rapidly switch between venom types with different proteome profiles in response to predatory or defensive stimuli. A novel conotoxin, GXIA (original name G117), belonging to the I3-subfamily was identified as the major component of the predatory venom of piscivorous Conus geographus. Using 2D solution NMR spectroscopy techniques, we resolved the 3D structure for GXIA, the first structure reported for the I3-subfamily and framework XI family. The 32 amino acid peptide is comprised of eight cysteine residues with the resultant disulfide connectivity forming an ICK+1 motif. With a triple stranded β-sheet, the GXIA backbone shows striking similarity to Citation: Armstrong, D.A.; Jin, A.-H.; several tarantula toxins targeting the voltage sensor of voltage gated potassium and sodium channels.