Intake, Apparent Digestibility, and Digesta Passage in Leopard Tortoises (Geochelone Pardalis ) Fed a Complete, Extruded Feed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Husbandry and Captive Breeding of the Bated by Slow Or Indirect Shipping
I\IJtIlD AND T'II'LT' Iil1,TUKTS j r8- I.r I o, ee+,, JilJilill fl:{:r;,i,,'J,:*,1;l: dehydration, and thermal exposure of improper or protracted warehousing on either or both sides of the Atlantic, exacer- Husbandry and Captive Breeding of the bated by slow or indirect shipping. These situations may also Parrot-Beaked Tortoise, have provided a dramatic stress-related increase in parasite Homopus areolatus or bacterial loads, or rendered pathogens more virulent, thereby adversely affecting the health and immunity levels Jnuns E. BIRZYKI of the tortoises. With this in mind, 6 field-collected tortoises | (3 project 530 l,{orth Park Roacl, Lct Grctnge Pcrrk, Illinois 60525 USA males, 3 females) used in this were airfreighted directly from South Africa to fully operational indoor cap- The range of the parrot-beaked tortoise, Hontopus tive facilities in the USA. A number of surplus captive areolatus, is restricted to the Cape Province in South Africa specirnens (7) were also available, and these were included (Loveridge and Williams, 1957; Branch, 1989). It follows in the study group. the coastline of the Indian Ocean from East London in the Materials ancl MethocLr. - Two habitat enclosures mea- east, around the Cape of Good Hope along the Atlantic surin-e 7 x2 feet (2.1 x 0.6 m) were constructed of plywood Ocean, north to about Clanwilliam. This ran-qe is bordered in and each housed two males and either four or five females. the northeast by the very arid Great Karoo, an area receiving These were enclosed on all sides, save for the front, which less than 250 mm of rain annually, and in the north by the had sliding ..elass doors. -

The Conservation Biology of Tortoises
The Conservation Biology of Tortoises Edited by Ian R. Swingland and Michael W. Klemens IUCN/SSC Tortoise and Freshwater Turtle Specialist Group and The Durrell Institute of Conservation and Ecology Occasional Papers of the IUCN Species Survival Commission (SSC) No. 5 IUCN—The World Conservation Union IUCN Species Survival Commission Role of the SSC 3. To cooperate with the World Conservation Monitoring Centre (WCMC) The Species Survival Commission (SSC) is IUCN's primary source of the in developing and evaluating a data base on the status of and trade in wild scientific and technical information required for the maintenance of biological flora and fauna, and to provide policy guidance to WCMC. diversity through the conservation of endangered and vulnerable species of 4. To provide advice, information, and expertise to the Secretariat of the fauna and flora, whilst recommending and promoting measures for their con- Convention on International Trade in Endangered Species of Wild Fauna servation, and for the management of other species of conservation concern. and Flora (CITES) and other international agreements affecting conser- Its objective is to mobilize action to prevent the extinction of species, sub- vation of species or biological diversity. species, and discrete populations of fauna and flora, thereby not only maintain- 5. To carry out specific tasks on behalf of the Union, including: ing biological diversity but improving the status of endangered and vulnerable species. • coordination of a programme of activities for the conservation of biological diversity within the framework of the IUCN Conserva- tion Programme. Objectives of the SSC • promotion of the maintenance of biological diversity by monitor- 1. -
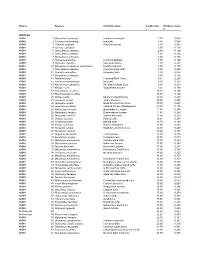
PDF File Containing Table of Lengths and Thicknesses of Turtle Shells And
Source Species Common name length (cm) thickness (cm) L t TURTLES AMNH 1 Sternotherus odoratus common musk turtle 2.30 0.089 AMNH 2 Clemmys muhlenbergi bug turtle 3.80 0.069 AMNH 3 Chersina angulata Angulate tortoise 3.90 0.050 AMNH 4 Testudo carbonera 6.97 0.130 AMNH 5 Sternotherus oderatus 6.99 0.160 AMNH 6 Sternotherus oderatus 7.00 0.165 AMNH 7 Sternotherus oderatus 7.00 0.165 AMNH 8 Homopus areolatus Common padloper 7.95 0.100 AMNH 9 Homopus signatus Speckled tortoise 7.98 0.231 AMNH 10 Kinosternon subrabum steinochneri Florida mud turtle 8.90 0.178 AMNH 11 Sternotherus oderatus Common musk turtle 8.98 0.290 AMNH 12 Chelydra serpentina Snapping turtle 8.98 0.076 AMNH 13 Sternotherus oderatus 9.00 0.168 AMNH 14 Hardella thurgi Crowned River Turtle 9.04 0.263 AMNH 15 Clemmys muhlenbergii Bog turtle 9.09 0.231 AMNH 16 Kinosternon subrubrum The Eastern Mud Turtle 9.10 0.253 AMNH 17 Kinixys crosa hinged-back tortoise 9.34 0.160 AMNH 18 Peamobates oculifers 10.17 0.140 AMNH 19 Peammobates oculifera 10.27 0.140 AMNH 20 Kinixys spekii Speke's hinged tortoise 10.30 0.201 AMNH 21 Terrapene ornata ornate box turtle 10.30 0.406 AMNH 22 Terrapene ornata North American box turtle 10.76 0.257 AMNH 23 Geochelone radiata radiated tortoise (Madagascar) 10.80 0.155 AMNH 24 Malaclemys terrapin diamondback terrapin 11.40 0.295 AMNH 25 Malaclemys terrapin Diamondback terrapin 11.58 0.264 AMNH 26 Terrapene carolina eastern box turtle 11.80 0.259 AMNH 27 Chrysemys picta Painted turtle 12.21 0.267 AMNH 28 Chrysemys picta painted turtle 12.70 0.168 AMNH 29 -

Conservation of South African Tortoises with Emphasis on Their Apicomplexan Haematozoans, As Well As Biological and Metal-Fingerprinting of Captive Individuals
CONSERVATION OF SOUTH AFRICAN TORTOISES WITH EMPHASIS ON THEIR APICOMPLEXAN HAEMATOZOANS, AS WELL AS BIOLOGICAL AND METAL-FINGERPRINTING OF CAPTIVE INDIVIDUALS By Courtney Antonia Cook THESIS submitted in fulfilment of the requirements for the degree PHILOSOPHIAE DOCTOR (Ph.D.) in ZOOLOGY in the FACULTY OF SCIENCE at the UNIVERSITY OF JOHANNESBURG Supervisor: Prof. N. J. Smit Co-supervisors: Prof. A. J. Davies and Prof. V. Wepener June 2012 “We need another and a wiser and perhaps a more mystical concept of animals. Remote from universal nature, and living by complicated artifice, man in civilization surveys the creature through the glass of his knowledge and sees thereby a feather magnified and the whole image in distortion. We patronize them for their incompleteness, for their tragic fate of having taken form so far below ourselves. And therein we err, and greatly err. For the animal shall not be measured by man. In a world older and more complete than ours they move finished and complete, gifted with extensions of the senses we have lost or never attained, living by voices we shall never hear. They are not brethren, they are not underlings; they are other nations caught with ourselves in the net of life and time, fellow prisoners of the splendour and travail of the earth.” Henry Beston (1928) ABSTRACT South Africa has the highest biodiversity of tortoises in the world with possibly an equivalent diversity of apicomplexan haematozoans, which to date have not been adequately researched. Prior to this study, five apicomplexans had been recorded infecting southern African tortoises, including two haemogregarines, Haemogregarina fitzsimonsi and Haemogregarina parvula, and three haemoproteids, Haemoproteus testudinalis, Haemoproteus balazuci and Haemoproteus sp. -

Homopus Research Foundation
Homopus Research Foundation Annual Report 2011 Victor Loehr January 2012 Homopus Research Foundation: annual report 2011 1 Homopus Research Foundation: annual report 2011 CONTENTS 1. INTRODUCTION AND ACHIEVEMENTS IN 2011 .......................................................................................... 4 1.1. LONG-TERM STUDBOOK MANAGEMENT PLAN HOMOPUS SIGNATUS .............................................. 5 1.2. PROGRESS LONG-TERM FIELD STUDY HOMOPUS FEMORALIS ....................................................... 6 2. PLANS FOR 2012 AND THEREAFTER ........................................................................................................ 6 3. STUDBOOK SUMMARIES ......................................................................................................................... 7 4. ACTUAL STUDBOOK OVERVIEWS ............................................................................................................. 8 5. SPECIFIC INFORMATION FROM STUDBOOK PARTICIPANTS ......................................................................... 20 6. NEW PUBLICATIONS ............................................................................................................................ 25 7. FINANCIAL REPORT ............................................................................................................................. 26 8. PERMIT OVERVIEW .............................................................................................................................. 26 APPENDIX 1 - MEETING REPORT -

REPRODUCTION of the SMALLEST TORTOISE, the NAMAQUALAND SPECKLED PADLOPER, HOMOPUS SIGNATUS SIGNATUS Author(S): Victor J
REPRODUCTION OF THE SMALLEST TORTOISE, THE NAMAQUALAND SPECKLED PADLOPER, HOMOPUS SIGNATUS SIGNATUS Author(s): Victor J. T. Loehr, Brian T. Henen, Margaretha D. Hofmeyr Source: Herpetologica, 60(4):444-454. 2004. Published By: The Herpetologists' League DOI: http://dx.doi.org/10.1655/03-59 URL: http://www.bioone.org/doi/full/10.1655/03-59 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Herpetologica, 60(4), 2004, 438–444 Ó 2004 by The Herpetologists’ League, Inc. HABITAT SELECTION BY SPHAERODACTYLUS NICHOLSI (SQUAMATA: GEKKONIDAE) IN CABO ROJO, PUERTO RICO 1 2,3 RICARDO LO´ PEZ-ORTIZ AND ALLEN R. LEWIS 1Departamento de Recursos Naturales y Ambientales de Puerto Rico, Negociado de Pesca y Vida Silvestre, P.O. Box 491 Boquero´n, Puerto Rico 00622 2Department of Biology, University of Puerto Rico, Mayagu¨ez, Puerto Rico 00681-9012 ABSTRACT: We studied habitat selection by the litter-dwelling gecko Sphaerodactylus nicholsi in the Cabo Rojo National Wildlife Refuge in southwestern Puerto Rico to determine how this small diurnal species exists in an apparently xeric habitat. -

Chelonian Advisory Group Regional Collection Plan 4Th Edition December 2015
Association of Zoos and Aquariums (AZA) Chelonian Advisory Group Regional Collection Plan 4th Edition December 2015 Editor Chelonian TAG Steering Committee 1 TABLE OF CONTENTS Introduction Mission ...................................................................................................................................... 3 Steering Committee Structure ........................................................................................................... 3 Officers, Steering Committee Members, and Advisors ..................................................................... 4 Taxonomic Scope ............................................................................................................................. 6 Space Analysis Space .......................................................................................................................................... 6 Survey ........................................................................................................................................ 6 Current and Potential Holding Table Results ............................................................................. 8 Species Selection Process Process ..................................................................................................................................... 11 Decision Tree ........................................................................................................................... 13 Decision Tree Results ............................................................................................................. -

Studbook Management Plan Homopus Areolatus
Common padloper Homopus areolatus Studbook Management Plan Version 4, November 2020 Victor Loehr Studbook Management Plan Homopus areolatus VERSION HISTORY Version Date Changes 1 (draft) April 2015 - 2 July 2015 Comments from studbook participants implemented 3 September 2020 Five-yearly update 4 November 2020 Comments from studbook participants implemented This plan will be reviewed and updated once every five years. Progress will be reported annually, in the annual reports of Dwarf Tortoise Conservation. 1 Studbook Management Plan Homopus areolatus CONTENTS 1. INTRODUCTION.................................................................................................................................................... 3 2. DISTRIBUTION .....................................................................................................................................................3 3. HABITAT ..............................................................................................................................................................3 4. PROTECTED STATUS ............................................................................................................................................3 5. CONSERVATION STATUS ......................................................................................................................................4 6. STATUS IN CAPTIVITY .......................................................................................................................................... 4 7. STUDBOOK -

Cop17 Doc. 73
Original language: English CoP17 Doc. 73 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________________ Seventeenth meeting of the Conference of the Parties Johannesburg (South Africa), 24 September - 5 October 2016 Species specific matters TORTOISES AND FRESHWATER TURTLES (TESTUDINES SPP.) 1. This document has been prepared by the Secretariat. Background 2. At its 16th meeting (CoP16, Bangkok, 2013), the Conference of the Parties adopted Decisions 16.109 to 16.124 on Tortoises and freshwater turtles (Testudines spp.), as follows: Directed to the Secretariat 16.109 Subject to external funding, the Secretariat shall contract independent consultants to undertake a study, taking into account the findings of the Cancún workshop on non-detriment findings and other pertinent sources of information, to identify and discuss factors that are of particular relevance to make non-detriment findings for tortoises and freshwater turtles. These factors should include, but not be limited to, tortoise and turtle population status and dynamics, trade dynamics, production systems, and trade in parts and derivatives. This study should provide guidance to make non-detriment findings for tortoises and freshwater turtles. 16.110 The Secretariat shall make the results of the study referred to in Decision 16.109 available to the Animals Committee for consideration, if possible, at its 27th meeting. Directed to the Animals Committee 16.111 The Animals Committee shall review the study undertaken in accordance with Decision 16.109 and make recommendations, as appropriate and, if possible, at its 27th meeting for consideration by the Standing Committee and the Parties. Directed to the Standing Committee 16.112 The Standing Committee shall review the study undertaken in accordance with Decision 16.109 and the Animals Committee recommendations, and make its own recommendations, as appropriate, for communication to the Parties or for consideration at the 17th meeting of the Conference of the Parties. -

ZOOLOGY Zoology 110 (2007) 298–307
ARTICLE IN PRESS ZOOLOGY Zoology 110 (2007) 298–307 www.elsevier.de/zool When genes meet nomenclature: Tortoise phylogeny and the shifting generic concepts of Testudo and Geochelone Uwe Fritza,Ã, Olaf R.P. Bininda-Emondsb aMuseum of Zoology, Natural History State Collections Dresden, A.B. Meyer Building, D-01109 Dresden, Germany bInstitut fu¨r Spezielle Zoologie und Evolutionsbiologie mit Phyletischem Museum, Friedrich-Schiller-Universita¨t Jena, Erbertstrasse 1, D-07743 Jena, Germany Received 14 December 2006; received in revised form 8 February 2007; accepted 9 February 2007 Abstract We used a five-gene data set (mtDNA: 12S rRNA, 16S rRNA, cyt-b; nDNA: Cmos, Rag2) comprising approximately two-thirds of all extant testudinid species and, for the first time, including all five Testudo species to investigate the question of whether all western Palaearctic testudinids are monophyletic. Further, we examined whether the recently suggested allocation of the African Geochelone pardalis in the otherwise exclusively South African genus Psammobates and of the Malagasy G. yniphora in the monotypic genus Angonoka is justified in the face of considerable morphological evidence against such placements. Our phylogenetic analyses do not support the paraphyly and generic break-up of Testudo, as suggested by previous papers using a smaller taxon sampling and mtDNA data only. We propose a continued usage of the generic name Testudo for all five western Palaearctic tortoise species. Within Testudo, two monophyletic subclades are present, one containing T. hermanni+T. horsfieldii, and the other comprising (T. kleinmanni+T. marginata)+T. graeca. Nomenclaturally, we demonstrate that Eurotestudo Lapparent de Broin et al., 2006, which was recently erected with the type species T. -

TSA Magazine 2009
AUGUST 2009 TSATURTLE SURVIVAL ALLIANCE Transforming passion for turtles into effective conservation action through a global network of living collections and recovery programs WWW.TURTLESURVIVAL.ORG The Northern Spider Tortoise, (Pyxis arachnoides brygooi) See Cover Story on p.5 Peter Praschag, Behler Chelonian Center 1 From the President's Desk Ability to respond rapidly is what sets TSA apart s I write this letter, we have just received news that the female Rafetus has laid her first clutch of 56 eggs at Suzhou Zoo for the 2009 Aseason. After suffering last year’s disappointing news, the TSA regrouped and is giving it 100%, addressing some of the problems seen in 2008. We hope to be able to report soon that the world’s population of Rafetus has grown by fourteen-fold and to show the first photographs of a live hatchling R. swinhoei. When Dr. Gerald Kuchling’s email message arrived with this much-anticipated news, he requested that we try and find funding for a camcorder to mount over theRafetus nesting beach that would make finding eggs easier. I forwarded the email to the new TSA Board and almost immediately David Shapiro responded from Hong Kong, offering up the $1,400 requested. It struck me later what an amazing example this was of the TSA network’s strength, and our ability to respond to turtle conservation issues around the globe. No other turtle conservation organization is positioned to respond as rapidly or effectively as the TSA. Another notable strength of the TSA is our global network of veterinarians that can mobilize quickly when situations demand. -

2018 Annual Report
O N S E C R E V L A T N R C U Y T T C TURTLE CONSERVANCY ✯ ANNUAL REPORT 2018 TURTLE CONSERVANCY POST OFFICE BOX 1289 • OJAI, CALIFORNIA 93024 (212) 353-5060 • WWW.TURTLECONSERVANCY.ORG 2018 ANNUAL REPORT CREDITS INSIDE FRONT COVER: Jean-Philippe Delhomme; PAGE 2: Jean-Philippe Delhomme; PAGE 3: Robert Legg; PAGES 6-7: Turtle Conservancy; PAGES 8-10: Simon Rouot; PAGES 12-13: Maximilian Maurer; PAGE 14: Simon Rouot; PAGE 16: Turtle Conservancy; PAGE 17: Simon Rouot; PAGE 18: James Liu; PAGE 19: Simon Rouot; PAGE 20: Nathanael Stanek; PAGE 21: James Liu, Dreef Atsimo Andrefana, Turtle Conservancy; PAGE 22: Simon Rouot; PAGE 23: James Juvik; PAGE 24: Simon Rouot; PAGE 25: Turtle Conservancy; PAGE 26: Simon Rouot; PAGE 27: James Liu; PAGE 28: Robert Legg; PAGES 29-30: Maximilian Maurer; PAGE 31: James Liu; PAGE 32: Turtle Conservancy; PAGE 36: Robert Legg; PAGE 37: Turtle Conservancy; INSIDE BACK COVER: Maximilian Maurer CONTENTS A WORD FROM OUR FOUNDER 2 MISSION STATEMENT AND OBJECTIVES 3 STAFF AND BOARD MEMBERS 4 Staff 4 Board of Directors 5 Honorary and Advisory Boards 6 IN-RANGE CONSERVATION 8 Mexico: Bolson Tortoise Ecosystem Preserve 10 South Africa: Geometric Tortoise Fynbos Ecosystem Preserve 14 Madagascar: Ploughshare Tortoise Conservation 17 Madagascar: Radiated Tortoise Trafficking Crisis 19 Namibia: Conservation Hope for the Nama Padloper 22 Philippines: Palawan Forest Turtle Conservation 24 United States: Terrapin Nesting Project 26 CONSERVATION BREEDING CENTER 28 Summary of Hatchlings 28 Updates to the Conservation Breeding Center 29 TRADE INTERDICTION 30 US Fish and Wildlife Service Confiscations 30 CITES Work for Tortoises and Freshwater Turtles 31 OUTREACH AND AWARENESS 32 Public Outreach 33 The Tortoise Magazine 34 Scientific Publications 35 CONSERVATION SCIENCE, RESEARCH AND POLICY 36 2018 FINANCIALS 38 A WORD FROM OUR FOUNDER Over the last few decades, technology has significantly transformed how humans in- teract with the world, and now, it transforms how we protect the world’s most endan- gered species.