The Molecular Systematics of Southern African Testudinidae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Husbandry and Captive Breeding of the Bated by Slow Or Indirect Shipping
I\IJtIlD AND T'II'LT' Iil1,TUKTS j r8- I.r I o, ee+,, JilJilill fl:{:r;,i,,'J,:*,1;l: dehydration, and thermal exposure of improper or protracted warehousing on either or both sides of the Atlantic, exacer- Husbandry and Captive Breeding of the bated by slow or indirect shipping. These situations may also Parrot-Beaked Tortoise, have provided a dramatic stress-related increase in parasite Homopus areolatus or bacterial loads, or rendered pathogens more virulent, thereby adversely affecting the health and immunity levels Jnuns E. BIRZYKI of the tortoises. With this in mind, 6 field-collected tortoises | (3 project 530 l,{orth Park Roacl, Lct Grctnge Pcrrk, Illinois 60525 USA males, 3 females) used in this were airfreighted directly from South Africa to fully operational indoor cap- The range of the parrot-beaked tortoise, Hontopus tive facilities in the USA. A number of surplus captive areolatus, is restricted to the Cape Province in South Africa specirnens (7) were also available, and these were included (Loveridge and Williams, 1957; Branch, 1989). It follows in the study group. the coastline of the Indian Ocean from East London in the Materials ancl MethocLr. - Two habitat enclosures mea- east, around the Cape of Good Hope along the Atlantic surin-e 7 x2 feet (2.1 x 0.6 m) were constructed of plywood Ocean, north to about Clanwilliam. This ran-qe is bordered in and each housed two males and either four or five females. the northeast by the very arid Great Karoo, an area receiving These were enclosed on all sides, save for the front, which less than 250 mm of rain annually, and in the north by the had sliding ..elass doors. -

Body Condition Assessment – As a Welfare and Management Assessment Tool for Radiated Tortoises (Astrochelys Radiata)
Body condition assessment – as a welfare and management assessment tool for radiated tortoises (Astrochelys radiata) Hullbedömning - som ett verktyg för utvärdering av välfärd och skötsel av strålsköldpadda (Astrochelys radiata) Linn Lagerström Independent project • 15 hp Swedish University of Agricultural Sciences, SLU Department of Animal Environment and Health Programme/Education Uppsala 2020 2 Body condition assessment – as a welfare and management tool for radiated tortoises (Astrochelys radiata) Hullbedömning - som ett verktyg för utvärdering av välfärd och skötsel av strålsköldpadda (Astrochelys radiata) Linn Lagerström Supervisor: Lisa Lundin, Swedish University of Agricultural Sciences, Department of Animal Environment and Health Examiner: Maria Andersson, Swedish University of Agricultural Sciences, Department of Animal Environment and Health Credits: 15 hp Level: First cycle, G2E Course title: Independent project Course code: EX0894 Programme/education: Course coordinating dept: Department of Aquatic Sciences and Assessment Place of publication: Uppsala Year of publication: 2020 Cover picture: Linn Lagerström Keywords: Tortoise, turtle, radiated tortoise, Astrochelys radiata, Geochelone radiata, body condition indices, body condition score, morphometrics Swedish University of Agricultural Sciences Faculty of Natural Resources and Agricultural Sciences Department of Animal Environment and Health 3 Publishing and archiving Approved students’ theses at SLU are published electronically. As a student, you have the copyright to your own work and need to approve the electronic publishing. If you check the box for YES, the full text (pdf file) and metadata will be visible and searchable online. If you check the box for NO, only the metadata and the abstract will be visiable and searchable online. Nevertheless, when the document is uploaded it will still be archived as a digital file. -

The Conservation Biology of Tortoises
The Conservation Biology of Tortoises Edited by Ian R. Swingland and Michael W. Klemens IUCN/SSC Tortoise and Freshwater Turtle Specialist Group and The Durrell Institute of Conservation and Ecology Occasional Papers of the IUCN Species Survival Commission (SSC) No. 5 IUCN—The World Conservation Union IUCN Species Survival Commission Role of the SSC 3. To cooperate with the World Conservation Monitoring Centre (WCMC) The Species Survival Commission (SSC) is IUCN's primary source of the in developing and evaluating a data base on the status of and trade in wild scientific and technical information required for the maintenance of biological flora and fauna, and to provide policy guidance to WCMC. diversity through the conservation of endangered and vulnerable species of 4. To provide advice, information, and expertise to the Secretariat of the fauna and flora, whilst recommending and promoting measures for their con- Convention on International Trade in Endangered Species of Wild Fauna servation, and for the management of other species of conservation concern. and Flora (CITES) and other international agreements affecting conser- Its objective is to mobilize action to prevent the extinction of species, sub- vation of species or biological diversity. species, and discrete populations of fauna and flora, thereby not only maintain- 5. To carry out specific tasks on behalf of the Union, including: ing biological diversity but improving the status of endangered and vulnerable species. • coordination of a programme of activities for the conservation of biological diversity within the framework of the IUCN Conserva- tion Programme. Objectives of the SSC • promotion of the maintenance of biological diversity by monitor- 1. -

Descriptions of Some A.Frican Tortoises
A"nals aftlte Natal Museum. Vol. VI, 1)(1ft 3 DESCRIPTIONS OF SOME AFRICAN TORTOISES. 46 1 Descriptions of some A.frican Tortoises. By John II C\\'itt. With P lates XXXVI-XXXVIII, and 5 Text. fi gul·es. CONTENTS. P elusios s inuatus s inu n.t us Smith J(j2 P elus ios s illu atus zuluensis H elQift 462 P ell1si os ni g r ica. ns n igricnns DonllC/. 4(\3 Pelus ios ni g ri can s castanoides SIIUS)1. n . 4(13 Peil1si os nigr icn.ns ca.stan e us Schw. 464 P elus ios nigricans rh odesianus H ellJi tt 465 Kinixys hellian!} Gray -I n? Kinixys nogll ey i Lataste ·HiS Kin ixys s pekii Gray. 469 Kinixys be lliana. zombensis sllbsp. '11. ·WO Kinixys belJin.nn. zulllon sis subs)1 . n. 47 1 Kinixys n. ustra lis 8p. It. 477 Kinixys da.rlingi Blgr. 4, 1 Kini xys jordn.ni sp . n . 482 Kinixys yonn gi ap. n. 4SG Kini xys lo bn.ts ia na Pou'er 488 H omopus WaMb. ..HHi Pseudomopus g. n . ·Hlli T estlld o L . 4!)H Neotestlldo g. n. 50" 462 JOHN HEWI'IT. THE material dealt wi th in this revi ew has bef' 1l derived from varioll s sources. A good portion of it is contained in the Albany Musemu, and for the loan of specimens I am especia.lly indebted to the Mu seums at Pretoria, Kimberley and Pietermaritzburg. It, may be .aid that the. present study again illustrates the fact t.hat the closer a group of organisms is studied and the wider the area from which they are obt.ained, the greater is the difficnlty ill formulating any clear diagnosis of specific characters. -
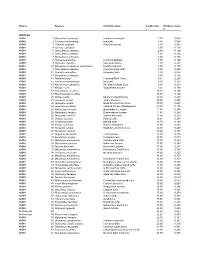
PDF File Containing Table of Lengths and Thicknesses of Turtle Shells And
Source Species Common name length (cm) thickness (cm) L t TURTLES AMNH 1 Sternotherus odoratus common musk turtle 2.30 0.089 AMNH 2 Clemmys muhlenbergi bug turtle 3.80 0.069 AMNH 3 Chersina angulata Angulate tortoise 3.90 0.050 AMNH 4 Testudo carbonera 6.97 0.130 AMNH 5 Sternotherus oderatus 6.99 0.160 AMNH 6 Sternotherus oderatus 7.00 0.165 AMNH 7 Sternotherus oderatus 7.00 0.165 AMNH 8 Homopus areolatus Common padloper 7.95 0.100 AMNH 9 Homopus signatus Speckled tortoise 7.98 0.231 AMNH 10 Kinosternon subrabum steinochneri Florida mud turtle 8.90 0.178 AMNH 11 Sternotherus oderatus Common musk turtle 8.98 0.290 AMNH 12 Chelydra serpentina Snapping turtle 8.98 0.076 AMNH 13 Sternotherus oderatus 9.00 0.168 AMNH 14 Hardella thurgi Crowned River Turtle 9.04 0.263 AMNH 15 Clemmys muhlenbergii Bog turtle 9.09 0.231 AMNH 16 Kinosternon subrubrum The Eastern Mud Turtle 9.10 0.253 AMNH 17 Kinixys crosa hinged-back tortoise 9.34 0.160 AMNH 18 Peamobates oculifers 10.17 0.140 AMNH 19 Peammobates oculifera 10.27 0.140 AMNH 20 Kinixys spekii Speke's hinged tortoise 10.30 0.201 AMNH 21 Terrapene ornata ornate box turtle 10.30 0.406 AMNH 22 Terrapene ornata North American box turtle 10.76 0.257 AMNH 23 Geochelone radiata radiated tortoise (Madagascar) 10.80 0.155 AMNH 24 Malaclemys terrapin diamondback terrapin 11.40 0.295 AMNH 25 Malaclemys terrapin Diamondback terrapin 11.58 0.264 AMNH 26 Terrapene carolina eastern box turtle 11.80 0.259 AMNH 27 Chrysemys picta Painted turtle 12.21 0.267 AMNH 28 Chrysemys picta painted turtle 12.70 0.168 AMNH 29 -

Movement, Home Range and Habitat Use in Leopard Tortoises (Stigmochelys Pardalis) on Commercial
Movement, home range and habitat use in leopard tortoises (Stigmochelys pardalis) on commercial farmland in the semi-arid Karoo. Martyn Drabik-Hamshare Submitted in fulfilment of the academic requirements for the degree of Master of Science in the Discipline of Ecological Sciences School of Life Sciences College of Agriculture, Engineering and Science University of KwaZulu-Natal Pietermaritzburg Campus 2016 ii ABSTRACT Given the ever-increasing demand for resources due to an increasing human population, vast ranges of natural areas have undergone land use change, either due to urbanisation or production and exploitation of resources. In the semi-arid Karoo of southern Africa, natural lands have been converted to private commercial farmland, reducing habitat available for wildlife. Furthermore, conversion of land to energy production is increasing, with areas affected by the introduction of wind energy, solar energy, or hydraulic fracturing. Such widespread changes affects a wide range of animal and plant communities. Southern Africa hosts the highest diversity of tortoises (Family: Testudinidae), with up to 18 species present in sub-Saharan Africa, and 13 species within the borders of South Africa alone. Diversity culminates in the Karoo, whereby up to five species occur. Tortoises throughout the world are undergoing a crisis, with at least 80 % of the world’s species listed at ‘Vulnerable’ or above. Given the importance of many tortoise species to their environments and ecosystems— tortoises are important seed dispersers, whilst some species produce burrows used by numerous other taxa—comparatively little is known about certain aspects relating to their ecology: for example spatial ecology, habitat use and activity patterns. -

Growing and Shrinking in the Smallest Tortoise, Homopus Signatus Signatus: the Importance of Rain
CORE Metadata, citation and similar papers at core.ac.uk Provided by Springer - Publisher Connector Oecologia (2007) 153:479–488 DOI 10.1007/s00442-007-0738-7 GLOBAL CHANGE AND CONSERVATION ECOLOGY Growing and shrinking in the smallest tortoise, Homopus signatus signatus: the importance of rain Victor J. T. Loehr · Margaretha D. Hofmeyr · Brian T. Henen Received: 21 November 2006 / Accepted: 21 March 2007 / Published online: 24 April 2007 © Springer-Verlag 2007 Abstract Climate change models predict that the range of dorso-ventrally, so a reduction in internal matter due to the world’s smallest tortoise, Homopus signatus signatus, starvation or dehydration may have caused SH to shrink. will aridify and contract in the next decades. To evaluate Because the length and width of the shell seem more rigid, the eVects of annual variation in rainfall on the growth of reversible bone resorption may have contributed to shrink- H. s. signatus, we recorded annual growth rates of wild age, particularly of the shell width and plastron length. individuals from spring 2000 to spring 2004. Juveniles Based on growth rates for all years, female H. s. signatus grew faster than did adults, and females grew faster than need 11–12 years to mature, approximately twice as long as did males. Growth correlated strongly with the amount of would be expected allometrically for such a small species. rain that fell during the time just before and within the However, if aridiWcation lowers average growth rates to the growth periods. Growth rates were lowest in 2002–2003, level of 2002–2003, females would require 30 years to when almost no rain fell between September 2002 and mature. -

Conservation of South African Tortoises with Emphasis on Their Apicomplexan Haematozoans, As Well As Biological and Metal-Fingerprinting of Captive Individuals
CONSERVATION OF SOUTH AFRICAN TORTOISES WITH EMPHASIS ON THEIR APICOMPLEXAN HAEMATOZOANS, AS WELL AS BIOLOGICAL AND METAL-FINGERPRINTING OF CAPTIVE INDIVIDUALS By Courtney Antonia Cook THESIS submitted in fulfilment of the requirements for the degree PHILOSOPHIAE DOCTOR (Ph.D.) in ZOOLOGY in the FACULTY OF SCIENCE at the UNIVERSITY OF JOHANNESBURG Supervisor: Prof. N. J. Smit Co-supervisors: Prof. A. J. Davies and Prof. V. Wepener June 2012 “We need another and a wiser and perhaps a more mystical concept of animals. Remote from universal nature, and living by complicated artifice, man in civilization surveys the creature through the glass of his knowledge and sees thereby a feather magnified and the whole image in distortion. We patronize them for their incompleteness, for their tragic fate of having taken form so far below ourselves. And therein we err, and greatly err. For the animal shall not be measured by man. In a world older and more complete than ours they move finished and complete, gifted with extensions of the senses we have lost or never attained, living by voices we shall never hear. They are not brethren, they are not underlings; they are other nations caught with ourselves in the net of life and time, fellow prisoners of the splendour and travail of the earth.” Henry Beston (1928) ABSTRACT South Africa has the highest biodiversity of tortoises in the world with possibly an equivalent diversity of apicomplexan haematozoans, which to date have not been adequately researched. Prior to this study, five apicomplexans had been recorded infecting southern African tortoises, including two haemogregarines, Haemogregarina fitzsimonsi and Haemogregarina parvula, and three haemoproteids, Haemoproteus testudinalis, Haemoproteus balazuci and Haemoproteus sp. -

Investigating the Thermal Biology and Behaviour of Captive Radiated Tortoises
MedDocs Publishers ISSN: 2639-4391 Journal of Veterinary Medicine and Animal Sciences Open Access | Research Article Investigating the Thermal Biology and Behaviour of Captive Radiated Tortoises Avraham Terespolsky; James Edward Brereton* University Centre Sparsholt, England *Corresponding Author(s): James Edward Brereton Abstract University Centre Sparsholt, Westley Lane, Sparsholt, Thermoregulation is integral to the maintenance of rep- Winchester, Hampshire, SO21 2NF, England. tile biological function and health, and therefore is a key Email: [email protected] area of investigation for herpetologists. To investigate the relationship between core body temperature and behav- iour, a behavioural study was conducted in which iButton data loggers were placed on a group of captive radiated tor- Received: Nov 09, 2020 toises (Astrochelys radiata) located at Sparsholt College’s Accepted: Jan 04, 2021 Animal Management Centre, in Hampshire, UK. Correla- tions between core body temperature and specific behav- Published Online: Jan 08, 2021 iours were covered. Body mass had a significant effect on Journal: Journal of Veterinary Medicine and Animal Sciences average core body temperature (P= <0.0001) with higher Publisher: MedDocs Publishers LLC average temperatures recorded in larger individuals over Online edition: http://meddocsonline.org/ longer periods. There was a significant positive relationship between mean body temperature and basking behaviour, Copyright: © Brereton JE (2021). This Article is (P= 0.001, r= 0.485), and a negative correlation between distributed under the terms of Creative Commons mean body temperature and feeding (P= 0.006, r= -0.155). Attribution 4.0 International License Temperature did not significantly affect the prevalence of any other behaviours, though a trend toward greater ex- pression of social behaviour, and fewer bouts of aggressive ramming, was observed when tortoises achieved higher body temperatures. -

Chersina Angulata) on Dassen Island, South Africa
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/220028468 Sexual disparity in activity patterns and time budgets of angulate tortoises (Chersina angulata) on Dassen Island, South Africa Article in African Zoology · October 2006 DOI: 10.3377/1562-7020(2006)41[224:SDIAPA]2.0.CO;2 CITATIONS READS 13 77 3 authors: Toby Keswick Brian Henen University of the Western Cape 116 PUBLICATIONS 1,379 CITATIONS 7 PUBLICATIONS 40 CITATIONS SEE PROFILE SEE PROFILE Margaretha Hofmeyr University of the Western Cape 87 PUBLICATIONS 664 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Headstarting Agassiz's Desert Tortoises View project Tortoise ecology and conservation View project All content following this page was uploaded by Toby Keswick on 24 July 2014. The user has requested enhancement of the downloaded file. Sexual disparity in activity patterns and time budgets of angulate tortoises (Chersina angulata) on Dassen Island, South Africa Toby Keswick, Brian T. Henen & Margaretha D. Hofmeyr* Chelonian Biodiversity and Conservation – Southern Africa, Department of Biodiversity and Conservation Biology, University of the Western Cape, Private Bag X17, Bellville, 7535 South Africa Received 13 September 2005. Accepted 11 January 2006 Behavioural frequencies and time budgets for male and female Chersina angulata were recorded in spring, September 2004. The daily activity of the population was 10.51 ± 0.42 h (mean ± CI), but individual males and females were in the open for 2.57 ± 1.12 h and 1.58 ± 1.44 h, respectively. Both sexes spent nearly 3.5 h per day basking with 90% of the basking time in the cover of sparse vegetation. -

The Metabolic Responses of Adult Angulate Tortoises
CIBTech Journal of Zoology ISSN: 2319–3883 (Online) An Open Access, Online International Journal Available at http://www.cibtech.org/cjz.htm 2016 Vol. 5 (2) May-August, pp.61-72/Setlalekgomo and Winter Research Article THE METABOLIC RESPONSES OF ADULT ANGULATE TORTOISE (CHERSINA ANGULATA) TO VARYING AMBIENT TEMPERATURES *Setlalekgomo M.R.1 and P.E.D. Winter2 1Department of Basic Sciences, Botswana University of Agriculture and Natural Resources, Private Bag 0027, Gaborone, Botswana 2Department of Zoology, Nelson Mandela Metropolitan University, Box 77000, Port Elizabeth, 6031, South Africa *Author for Correspondence ABSTRACT Various physiological processes of ectotherms increase with increasing temperature. The effects of varying ambient temperature and gender on the metabolic rate (MR) of adult Chersina angulata were investigated. The tortoises were acclimated to 22 ± 1°C and a 14L:10D photoperiod in an environmentally controlled room prior to MR determination. Oxygen consumption (VO2) rates were determined as index of MR in fasted and resting adult tortoises at eight experimental temperatures (14°C, 18°C, 22°C, 26°C, 30°C, 35°C, 38°C and 40°C). Gender had no significant influence on the MR of C. angulata. MR increased with increasing temperature, but not consistently over the whole range of temperatures tested. MR was strongly temperature dependent, with a Q10 value of 4.13 between 14°C and 26°C. A plateau was detected within a temperature range of 26°C–38°C with a Q10 value of 1.13. The plateau may be a thermal preferendum for C. angulata which may render them advantage to forage at summer temperatures in their natural habitat in the Eastern Cape. -

Annual Report 2016 Homopus Research Foundation
Homopus Research Foundation Annual Report 2016 Victor Loehr February 2017 Homopus Research Foundation: annual report 2016 CONTENTS 1. INTRODUCTION AND ACHIEVEMENTS IN 2016 ........................................................................................ 2 1.1. POLICIES AND PERMANENT ACTION POINTS .................................................................................................. 2 1.2. OUTSTANDING ACTION POINTS FROM THE 2015 ANNUAL REPORT ................................................................... 2 1.3. STUDBOOK MANAGEMENT PLAN HOMOPUS SIGNATUS .................................................................................. 4 1.4. STUDBOOK MANAGEMENT PLAN HOMOPUS AREOLATUS ................................................................................ 5 1.5. PROGRESS FIELD STUDIES ON HOMOPUS .................................................................................................... 5 2. PLANS FOR 2017 AND THEREAFTER ..................................................................................................... 6 3. STUDBOOK SUMMARIES ...................................................................................................................... 6 4. ACTUAL STUDBOOK OVERVIEWS .......................................................................................................... 8 5. SPECIFIC INFORMATION FROM STUDBOOK PARTICIPANTS ....................................................................... 19 6. NEW PUBLICATIONS ........................................................................................................................