The Comparative Ecology of Waterfowl in North Queensland
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A 2010 Supplement to Ducks, Geese, and Swans of the World
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Ducks, Geese, and Swans of the World by Paul A. Johnsgard Papers in the Biological Sciences 2010 The World’s Waterfowl in the 21st Century: A 2010 Supplement to Ducks, Geese, and Swans of the World Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/biosciducksgeeseswans Part of the Ornithology Commons Johnsgard, Paul A., "The World’s Waterfowl in the 21st Century: A 2010 Supplement to Ducks, Geese, and Swans of the World" (2010). Ducks, Geese, and Swans of the World by Paul A. Johnsgard. 20. https://digitalcommons.unl.edu/biosciducksgeeseswans/20 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Ducks, Geese, and Swans of the World by Paul A. Johnsgard by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. The World’s Waterfowl in the 21st Century: A 200 Supplement to Ducks, Geese, and Swans of the World Paul A. Johnsgard Pages xvii–xxiii: recent taxonomic changes, I have revised sev- Introduction to the Family Anatidae eral of the range maps to conform with more current information. For these updates I have Since the 978 publication of my Ducks, Geese relied largely on Kear (2005). and Swans of the World hundreds if not thou- Other important waterfowl books published sands of publications on the Anatidae have since 978 and covering the entire waterfowl appeared, making a comprehensive literature family include an identification guide to the supplement and text updating impossible. -

Waterlines-74 National Waterbird Assessment
National waterbird assessment RT Kingsford, JL Porter and SA Halse Waterlines Report Series No. 74, March 2012 Waterlines This paper is part of a series of works commissioned by the National Water Commission on key water issues. This work has been undertaken by the Australian Wetlands and Rivers Centre of the University of New South Wales on behalf of the National Water Commission. © Commonwealth of Australia 2012 This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission. Requests and enquiries concerning reproduction and rights should be addressed to the Communications Director, National Water Commission, 95 Northbourne Avenue, Canberra ACT 2600 or email [email protected]. Online/print: ISBN: 978-1-921853-58-6 National waterbird assessment, March 2012 Authors: RT Kingsford, JL Porter and SA Halse Published by the National Water Commission 95 Northbourne Avenue Canberra ACT 2600 Tel: 02 6102 6000 Email: [email protected] Date of publication: March 2012 Cover design by: Angelink Front cover image courtesy of Prof RT Kingsford An appropriate citation for this report is: Kingsford RT, Porter JL and Halse SA 2011, National waterbird assessment, Waterlines report, National Water Commission, Canberra Disclaimer This paper is presented by the National Water Commission for the purpose of informing discussion and does not necessarily reflect the views or opinions of the Commission. Contents Foreword ix Acknowledgements x Executive summary xi 1. Introduction 1 1.1. Background 2 1.2. Project objectives 11 1.3. Report structure 11 2. -

Nature Terri Tory
NATURE TERRITORY November 2017 Newsletter of the Northern Territory Field Naturalists' Club Inc. In This Issue New Meeting Room p. 2 November Meeting p. 3 November Field Trip p. 4 October Field Trip Report p. 5 Upcoming Activities p. 6 Bird of the Month p. 7 Club notices p. 8 Club web-site: http://ntfieldnaturalists.org.au/ This photograph, entitled ?Bush Stone-curlew in Hiding?, was Runner-up in the Fauna category 2017 Northern Territory Field Naturalists? Club Wildlife Photograph Competition. Its story is on page 2 in this newsletter. Photo: Janis Otto. FOR THE DIARY November Meeting: Wed 8 Nov, 7.45 pm - "Island Arks" for Conservation of Endangered Species - Chris Jolly November Field Trip: 11-12 Nov - Bio-blitz at Mary River - Diana Lambert - See pages 3 and 4 for m ore det ails - Disclaimer: The views expressed in Nature Territory are not necessarily those of the NT Field Naturalists' Club Inc. or members of its Committee. Field Nat Meetings at New Location! Field Nats monthly meetings are now going to be held at Blue 2.1.51 - still at Charles Darwin University. Study the map below before you venture out on 8 November for our talk with Chris Jolly on "Island Arks" for conservation of Endangered Species. 2017 Northern Territory Field Naturalists? Club Wildlife Photograph Competition Fauna category: Runner-up Janis Otto. Here is the story behind Janis? photograph titled ?Bush Stone-curlew in Hiding? reproduced with her permission on the front cover of this newsletter: ?Although they look quite comical with their gangly gait and outstretched neck when running and flying, Bush Stone-curlews (Burhinus grallarius) actually give me the impression they are quite series creatures. -

Population Sizes of Cotton Pygmy-Goose Nettapus Coromandelianus Coromandelianus Gmelin in Some Places of Assam (India)
International Journal of Biodiversity and Conservation Vol. 4(1), pp. 15-21, January 2012 Available online http://www.academicjournals.org/IJBC DOI: 10.5897/IJBC10.097 ISSN 2141-243X ©2012 Academic Journals Full Length Research Paper Population sizes of cotton pygmy-goose Nettapus coromandelianus coromandelianus Gmelin in some places of Assam (India) Upadhyaya S.* and Saikia P. K. Department of Zoology, T. H. B. College, Karchantola, Sonitpur (Assam), PIN-784189, India. Accepted 29 November, 2011 The breeding season is of paramount importance to the population size of waterfowl. All of the increase in population size occurs, as does much of the mortality during that season, which is often but a fraction of the entire year. Population of a species is indicative of the environmental factors of that area which strictly influences the species. The study includes the population size of the Cotton Pygmy- goose Nettapus coromandelianus coromandelianus Gmelin, a least concern anatid of South-east Asia and near relatives of Pygmy-goose of New South Wales which is under threatened condition. The study also includes the parameters influencing the population sizes. Two hundred and three (203) Cotton Pygmy-geese were observed during breeding season in 2006 and 2009 in study sites of Assam. Adult survival rates were found high 0.99 to 0.94 (SE < 0.005), but duckling survival was much lower, varying from approximately 0.1 to 0.3 during 2007 to 2009. No naturally died specimens have been collected during the study period, but 7 specimens have been reported to be killed by hunters among a total of 22 gooses were killed during 2006 to 2008. -

2.9 Waterbirds: Identification, Rehabilitation and Management
Chapter 2.9 — Freshwater birds: identification, rehabilitation and management• 193 2.9 Waterbirds: identification, rehabilitation and management Phil Straw Avifauna Research & Services Australia Abstract All waterbirds and other bird species associated with wetlands, are described including how habitats are used at ephemeral and permanent wetlands in the south east of Australia. Wetland habitat has declined substantially since European settlement. Although no waterbird species have gone extinct as a result some are now listed as endangered. Reedbeds are taken as an example of how wetlands can be managed. Chapter 2.9 — Freshwater birds: identification, rehabilitation and management• 194 Introduction such as farm dams and ponds. In contrast, the Great-crested Grebe is usually associated with large Australia has a unique suite of waterbirds, lakes and deep reservoirs. many of which are endemic to this, the driest inhabited continent on earth, or to the Australasian The legs of grebes are set far back on the body region with Australia being the main stronghold making them very efficient swimmers. They forage for the species. Despite extensive losses of almost completely underwater pursuing fish and wetlands across the continent since European aquatic arthropods such as insects and crustaceans. settlement no extinctions of waterbirds have They are strong fliers but are poor at manoeuvering been recorded from the Australian mainland as in flight and generally prefer to dive underwater a consequence. However, there have been some to escape avian predators or when disturbed by dramatic declines in many populations and several humans. Flights between wetlands, some times species are now listed as threatened including over great distances, are carried out under the cover the Australasian Bittern, Botaurus poiciloptilus of darkness when it is safe from attack by most (nationally endangered). -

A Preliminary Risk Assessment of Cane Toads in Kakadu National Park Scientist Report 164, Supervising Scientist, Darwin NT
supervising scientist 164 report A preliminary risk assessment of cane toads in Kakadu National Park RA van Dam, DJ Walden & GW Begg supervising scientist national centre for tropical wetland research This report has been prepared by staff of the Environmental Research Institute of the Supervising Scientist (eriss) as part of our commitment to the National Centre for Tropical Wetland Research Rick A van Dam Environmental Research Institute of the Supervising Scientist, Locked Bag 2, Jabiru NT 0886, Australia (Present address: Sinclair Knight Merz, 100 Christie St, St Leonards NSW 2065, Australia) David J Walden Environmental Research Institute of the Supervising Scientist, GPO Box 461, Darwin NT 0801, Australia George W Begg Environmental Research Institute of the Supervising Scientist, GPO Box 461, Darwin NT 0801, Australia This report should be cited as follows: van Dam RA, Walden DJ & Begg GW 2002 A preliminary risk assessment of cane toads in Kakadu National Park Scientist Report 164, Supervising Scientist, Darwin NT The Supervising Scientist is part of Environment Australia, the environmental program of the Commonwealth Department of Environment and Heritage © Commonwealth of Australia 2002 Supervising Scientist Environment Australia GPO Box 461, Darwin NT 0801 Australia ISSN 1325-1554 ISBN 0 642 24370 0 This work is copyright Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the Supervising Scientist Requests and inquiries concerning reproduction -

A New Anseriform Genus and Species from the Nebraska Pliocene
A NEW ANSERIFORM GENUS AND SPECIES FROM THE NEBRASKA PLIOCENE LESTER L. SHORT AMONGavian fossilson loan to me from the University of Nebraska State Museumis the tarsometatarsusof a goose-likeanseriform bird from the early Plioceneof Nebraska. The tarsometatarsushas somefeatures of geeseand swans,and of the anatinetribe Tadornini,but it alsotends somewhattoward the Cairinini in someof its features. Comparisonwith extant and fossilAnseriformes in the American Museum of Natural History and the United States National Museum and a study of the literature have convincedme that this tarsometatarsusrepresents an undescribed speciesthat is sufficientlydistinct to warrant placementin a new genus. I thank the authoritiesof the above-mentionedmuseum for their help in conductingmy studies.I am gratefulto CharlesG. Sibley,who originally borrowedthe fossil,for permissionto studyit. It is a pleasureto acknowl- edgethe aid of C. B. Schultzfor the loan of the material, and H. B. Gundersonof the Universityof NebraskaState Museumfor usefulinfor- mationconcerning the fossil. The use and potential importance of stereophotographyin avian paleontologyhas beendiscussed by Cracraft (1968: 3-4). I hope the stereophotographsreproduced here will facilitatecomparisons by avian paleontologists. Heteroehen, new genus Type of genus.--Heterochenpratensis, new species. Diagnosis.--Anseriformtarsometatarsus, near the size of Anser anser, and characterizedby: trochleaenot spreadgreatly as in modern swans, geeseand sheldrakes;outer surfacegently curving toward distal end -

Kakadu National Park Management Plan 2007–2014 Akadu Is Aboriginal Kland
Kakadu Board of Management Kakadu National Park MANAGEMENT PLAN 2007–2014 PLAN MANAGEMENT KAKADU NATIONAL PARK MANAGEMENT PLAN 2007–2014 Design Direction Design 3888 Photos Comb-crested Jacana: Michael Nelson Magpie Goose: Greg Miles Orchid: Michael Nelson Termite mound: Michael Nelson Darter: Michael Nelson Lotus flower: Michael Nelson Fire management: Michael Nelson Tourists at Ubirr art site: Michael Nelson Paperbark trees: Michael Nelson Tourists at Ubirr lookout: Peter Wellings West Alligator Head main beach: Greg Miles 4WD at Gunlom Falls: Michael Nelson Artwork Guided walk: Emily Scheibe Ranger at rock art site: Kristina Williams Fire management: Rhiannon Compton Ranger in boat: Justin Giumelli Pandanus and tree: William Suitor Chapter Pages Photos: Ian Oswald-Jacobs Artwork Lotus flowers and birdlife (page 1): Kodi Nadji Water lily leaves on wetland (page 15): Gail Rotumah Crocodile and landscape (page 17): Curtis Yarrbar Park use – featuring Frilled lizard (page 152): Christine Marie Alangate Designed by Design Direction Printed by CanPrint on Monza Satin (100 per cent recycled stock, from plantation timber) Kakadu National Park MANAGEMENT PLAN 2007–2014 © director of national Parks 2007 iSBn: 978 06 42 55 33 94 this work is copyright. apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process, re-used or redistributed without prior written permission from the director of national Parks. any permitted reproduction must acknowledge the source of any such material reproduced and include a copy of the original copyright notice. requests and enquiries concerning reproduction and copyright should be addressed to: the assistant Secretary Parks australia north gPo Box 1260 darwin nt 0801 director of national Parks australian business number: 13051 694 963 this management Plan provides the general public and Park users/visitors with information about how the Park will be managed for the next seven years. -

2017 Tropical Australia Species List
Tropical Australia 2017 Tours Leaders: Eagle Eye Tours Barry Davies and Rob Elvish Common Name Scientific Name Seen/ Heard ANSERIFORMES: Anseranatidae 1 Magpie Goose Anseranas semipalmata s ANSERIFORMES: Anatidae 2 Plumed Whistling-Duck Dendrocygna eytoni s 3 Wandering Whistling-Duck Dendrocygna arcuata s 4 Radjah Shelduck Tadorna radjah s 5 Green Pygmy-Goose Nettapus pulchellus s 6 Pacific Black Duck Anas superciliosa s 7 Gray Teal Anas gracilis s 8 White-eyed Duck Aythya australis s PODICIPEDIFORMES: Podicipedidae 9 Australasian Grebe Tachybaptus novaehollandiae s CICONIIFORMES: Ciconiidae 10 Black-necked Stork Ephippiorhynchus asiaticus s SULIFORMES: Phalacrocoracidae 11 Little Black Cormorant Phalacrocorax sulcirostris s 12 Little Pied Cormorant Phalacrocorax melanoleucos s SULIFORMES: Anhingidae 13 Australasian Darter Anhinga novaehollandiae s PELECANIFORMES: Pelecanidae 14 Australian Pelican Pelecanus conspicillatus s PELECANIFORMES: Ardeidae 15 Pacific Heron Ardea pacifica s 16 Eastern Great Heron Ardea modesta s 17 Intermediate Egret Ardea intermedia s 18 White-faced Heron Egretta novaehollandiae s 19 Little Egret Egretta garzetta s 20 Pied Heron Egretta picata s 21 Cattle Egret Bubulcus ibis s 22 Striated Heron Butorides striata s PELECANIFORMES: Threskiornithidae 23 Glossy Ibis Plegadis falcinellus s 24 Australian Ibis Threskiornis moluccus s 25 Straw-necked Ibis Threskiornis spinicollis s 26 Royal Spoonbill Platalea regia s ACCIPITRIFORMES: Accipitridae Page 1 of 7 Tropical Australia 2017 Tours Leaders: Eagle Eye Tours Barry -

A Partial Classification of Waterfowl (Anatidae) Based on Single-Copy Dna
A PARTIAL CLASSIFICATION OF WATERFOWL (ANATIDAE) BASED ON SINGLE-COPY DNA CORT S. MADSEN, KEVIN P. MCHUGH, AND SIWO R. DE KLOET Instituteof MolecularBiophysics, The Florida State University, Tallahassee, Florida 32306 USA ABSTR^CT.--Single-copyDNA-DNA hybridizationwas used to establishphylogenetic re- lationshipsamong 13 speciesof waterfowl (Anatidae) chosenfrom 10 tribes. On the basisof UPGMA clusteringof ATtodistances, we suggestthat the tribes Anatini, Aythyini, Tadornini, Mergini, and Cairinini diverged more recently than the Anserini, Stictonettini,Oxyurini, Dendrocygnini,and Anseranatini.The FreckledDuck (Stictonettanaevosa, tribe Stictonettini) is only distantlyrelated to the other Anatidae.Presumably the lineage divergedvery early. The sheldgeese(tribe Tadornini) and the true geese(Anserini) are only remotely related. The Oxyurini, consideredto be in the subfamilyAnatinae, is remotely related to the other Anatidae. The Dendrocygnini form an isolatedtribe with no closerelationship to the swans and geese(subfamily Anserinae). We found that the screamers(Anhimidae) are distantly related to the Anatidae. A method to estimate missing cells in a data matrix of pairwise distancesis presented. ReceivedI May 1987,accepted 16 February1988. RECENTLY,nucleic acid sequenceanalysis has up about 10-40% of the total DNA (Eden et al. been usedto estimaterelationships of many or- 1978) but containsonly a small percentageof ganisms. The analysis can include direct se- the total sequencediversity. The remaining60- quencing of individual cloned genes -

Endangered Species (Import and Export) Act (Chapter 92A)
1 S 23/2005 First published in the Government Gazette, Electronic Edition, on 11th January 2005 at 5:00 pm. NO.S 23 ENDANGERED SPECIES (IMPORT AND EXPORT) ACT (CHAPTER 92A) ENDANGERED SPECIES (IMPORT AND EXPORT) ACT (AMENDMENT OF FIRST, SECOND AND THIRD SCHEDULES) NOTIFICATION 2005 In exercise of the powers conferred by section 23 of the Endangered Species (Import and Export) Act, the Minister for National Development hereby makes the following Notification: Citation and commencement 1. This Notification may be cited as the Endangered Species (Import and Export) Act (Amendment of First, Second and Third Schedules) Notification 2005 and shall come into operation on 12th January 2005. Deletion and substitution of First, Second and Third Schedules 2. The First, Second and Third Schedules to the Endangered Species (Import and Export) Act are deleted and the following Schedules substituted therefor: ‘‘FIRST SCHEDULE S 23/2005 Section 2 (1) SCHEDULED ANIMALS PART I SPECIES LISTED IN APPENDIX I AND II OF CITES In this Schedule, species of an order, family, sub-family or genus means all the species of that order, family, sub-family or genus. First column Second column Third column Common name for information only CHORDATA MAMMALIA MONOTREMATA 2 Tachyglossidae Zaglossus spp. New Guinea Long-nosed Spiny Anteaters DASYUROMORPHIA Dasyuridae Sminthopsis longicaudata Long-tailed Dunnart or Long-tailed Sminthopsis Sminthopsis psammophila Sandhill Dunnart or Sandhill Sminthopsis Thylacinidae Thylacinus cynocephalus Thylacine or Tasmanian Wolf PERAMELEMORPHIA -
Eton Range Realignment Project ATTACHMENT 2 to EPBC Ref: 2015/7552 Preliminary Documentation Residual Impact Assessment and Offset Proposal - 37
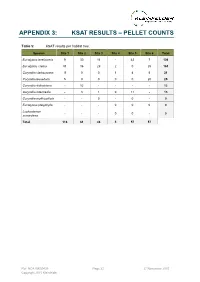
APPENDIX 3: KSAT RESULTS – PELLET COUNTS Table 5: KSAT results per habitat tree. Species Site 1 Site 2 Site 3 Site 4 Site 5 Site 6 Total Eucalyptus tereticornis 9 30 16 - 42 7 104 Eucalyptus crebra 91 16 29 2 0 25 163 Corymbia clarksoniana 11 0 0 1 4 5 21 Corymbia tessellaris 5 0 0 0 0 20 25 Corymbia dallachiana - 12 - - - - 12 Corymbia intermedia - 3 1 0 11 - 15 Corymbia erythrophloia - - 0 - 0 - 0 Eucalyptus platyphylla - - - 0 0 0 0 Lophostemon - - - 0 0 - 0 suaveolens Total 116 61 46 3 57 57 Ref: NCA15R30439 Page 22 27 November 2015 Copyright 2015 Kleinfelder APPENDIX 4: SITE PHOTOS The following images were taken from the centre of each BioCondition quadrat and represent a north east south west aspect, top left to bottom right. Ref: NCA15R30439 Page 23 27 November 2015 Copyright 2015 Kleinfelder Plate 3: BioCondition quadrat 1 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 24 27 November 2015 Copyright 2015 Kleinfelder Plate 4: BioCondition quadrat 2 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 25 27 November 2015 Copyright 2015 Kleinfelder Plate 5: BioCondition quadrat 3 (RE11.12.3) Ref: NCA15R30439 Page 26 27 November 2015 Copyright 2015 Kleinfelder Plate 6: BioCondition quadrat 4 (RE11.3.9) Ref: NCA15R30439 Page 27 27 November 2015 Copyright 2015 Kleinfelder Plate 7: BioCondition quadrat 5 (RE11.3.25) Ref: NCA15R30439 Page 28 27 November 2015 Copyright 2015 Kleinfelder Plate 8: BioCondition quadrat 6 (RE11.12.3/11.3.4/11.3.9) Ref: NCA15R30439 Page 29 27 November 2015 Copyright 2015 Kleinfelder Appendix E: Desktop Assessment for Potential