The Isotopic Chemical and Dissolved Gas Concentrations in Groundwater
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thusong — Bringing Hope to Small Towns
THUSONG — BRINGING HOPE TO SMALL TOWNS Small towns such as Burgersdorp, Oviston, Steynsburg and Venterstad subsist off the beaten track. The local Community Wwork Programme is creating work opportunities and stimulating entrepreneurship in an area where unemployment if rife – and even boosting the coffers of the local municipality. There is a stark beauty to the Free State and northern Within many homesteads trenches have been Eastern Cape. It’s a part of South Africa that has ploughed, ready to be sown with vegetables and grain. inspired thousands of paintings – a vast rural The local primary school has been completely landscape with potholed roads rolling down re-painted and re-furbished, and feels like a pleasant escarpments into countless plateaus, punctuated by environment where learners can be proud to study. windmills, mountain ranges and the occassional Before the advent of CWP, the members here would farmhouse either fish for subsistence or “do nothing”. Settlements are few and far between. Neighbours can The knock-on effects of the CWP programme in the be many kilometers away. Small forgotten towns such Thusong site is clearly evident. Gideon Mapete, Unit as Burgersdorp, Oviston, Steynsburg and and Manager of Steynsburg municipality and CWP Venterstad subsist quietly out of sight. This was once municipal contact, can undoubtedly see the positives. burgeoning farm country, full of grazing livestock. Not only has there been a small dent made into the From a distance the townships and towns are distinct unemployment rates, but also a small increase in the income of local government. As CWP members and clear. Iron sheets of RDP housing reflect the harsh sun on one side of the road, while a are receiving regular income, more are paying for neighbouring hamlet rests in the shade of large trees municipal services, therefore growing coffers and on the other. -

PUBLIC NOTICE No: 03/2020
PUBLIC NOTICE No: 03/2020 PUBLIC NOTICE: MID-YEAR BUDGET ISAZISO KULUNTU: INGXELO AND PERFORMANCE ASSESSMENT YOHLAHLO LWABIWO MALI REPORT FOR THE 2019/20 NENKCITHO KUNYAKA MALI KA FINANCIAL YEAR 2019/20 Notice is hereby given in terms of Oku kukwazisa ukuba ngokomhlathi Section 5 4 (1) of the Local Government wama 54 (1) ngokomthetho wolawulo Municipal Budget and Reporting lwezimali nen gxelo yomgaqo wonyaka ka Regulations, 2008 that the Honourable 2008 ku Rhulumente wasemakhaya, Executive Mayor of the Joe Gqabi District uSodolophu womasipala wesithili iJoe Municipality, Councillor Z.I. Dumzela, has Gqabi, uZI Dumzela, uthe thaca tabled in the Municipal Council the Mid- kwibhunga likamasipala ingxelo year Budget and Performance yesiqingatha sonyaka kuhlahlo lwabiwo Assess ment Report of the Joe Gqabi mali nenkcitho yalo masipala, kwakunye District Municipality and the Joe Gqabi nequmrhu lophuhliso eli bizwa JoGEDA Economic Development Agency kumnyaka ka 2019/20 (JoGEDA) for the 2019/20 financial year. Imiqulu engalengxelo iyafumaneka Copies of the documents are available kwiofisi zikamasipala wesithili eF79, Cnr for scrutiny during office hours at the Joe Cole and Graham Street, Barkly East Gqabi District Municipal offices, o ffice 9786. nakwezinye iofisi zalomasipala F79, Cnr Cole and Graham Street, Barkly ezikwezidolophu zilandelayo: Aliwal East, 9786. District satelite offices: North, Burgersdorp, Venterstad, Aliwal North, Burgersdorp, Venterstad, Steynsburg, Sterkspruit, Maclear, Ugie Steynsburg, Sterkspruit, Maclear, Ugie nase Mt Fletcher, kwiofisi zikamasipala and Mt Fletcher, Local Municipalities (Elundini, Senqu, Walter Sisulu , (Elundini, Senqu, Walter Sisulu, kumathala ogcino ncwadi Libraries as well a s on the District kwakwezidolophu zingentla nakwi website: www.jgdm.gov.za . For any website yethu ethi: www.jgdm.gov.za . -

SFD Promotion Initiative Ugie
SFD Promotion Initiative Ugie Elundini Local Municipality, Joe Gqabi District Municipality Eastern Cape, South Africa SFD Lite Final Report This SFD Lite Report was created through field-based research by Emanti Management and Centre for Science and Environment for a Water Research Commission project and as part of the SFD Promotion Initiative. Date of production: 3 October 2018 Last update: 22 October 2018 Ugie Produced by: Executive Summay South Africa EMANTI-CSE SFD Lite Report The SFD Promotion Initiative (SFD PI) has developed recommended methods and tools for preparing SFD Graphics and Reports. A full SFD Report consists of the SFD Graphic, the analysis of the service delivery context and enabling environment for service provision in the city for which you are preparing your SFD, and the complete record of data sources used. This analysis allows a systemic understanding of excreta management in the city, with evidence to support it. As a starting point (first step stone) to this (explained in detail in the SFD Manual), the SFD Lite is a simplified reporting template that summarises the key information about the excreta management situation in the city. SFD Lite Report Ugie, South Africa, 2018 Produced by: Unathi Jack, Emanti Philip de Souza, Emanti Shantanu Kumar Padhi, CSE Amrita Bhatnagar, CSE ©Copyright The tools and methods for SFD production were developed by the SFD Promotion Initiative and are available from: www.sfd.susana.org. All SFD materials are freely available following the open-source concept for capacity development and non-profit use, so long as proper acknowledgement of the source is made when used. -

Explore the Eastern Cape Province
Cultural Guiding - Explore The Eastern Cape Province Former President Nelson Mandela, who was born and raised in the Transkei, once said: "After having travelled to many distant places, I still find the Eastern Cape to be a region full of rich, unused potential." 2 – WildlifeCampus Cultural Guiding Course – Eastern Cape Module # 1 - Province Overview Component # 1 - Eastern Cape Province Overview Module # 2 - Cultural Overview Component # 1 - Eastern Cape Cultural Overview Module # 3 - Historical Overview Component # 1 - Eastern Cape Historical Overview Module # 4 - Wildlife and Nature Conservation Overview Component # 1 - Eastern Cape Wildlife and Nature Conservation Overview Module # 5 - Nelson Mandela Bay Metropole Component # 1 - Explore the Nelson Mandela Bay Metropole Module # 6 - Sarah Baartman District Municipality Component # 1 - Explore the Sarah Baartman District (Part 1) Component # 2 - Explore the Sarah Baartman District (Part 2) Component # 3 - Explore the Sarah Baartman District (Part 3) Component # 4 - Explore the Sarah Baartman District (Part 4) Module # 7 - Chris Hani District Municipality Component # 1 - Explore the Chris Hani District Module # 8 - Joe Gqabi District Municipality Component # 1 - Explore the Joe Gqabi District Module # 9 - Alfred Nzo District Municipality Component # 1 - Explore the Alfred Nzo District Module # 10 - OR Tambo District Municipality Component # 1 - Explore the OR Tambo District Eastern Cape Province Overview This course material is the copyrighted intellectual property of WildlifeCampus. -

Impact of Intergrating Teebus Hydro
IMPACT OF INTERGRATING TEEBUS HYDRO POWER ON THE UNBALANCED DISTRIBUTION MV NETWORK By: LINDANI MTHETHWA (213486806) A research dissertation submitted in fulfilment of the requirements for the degree of Masters in Technology: Engineering: Electrical in the Faculty of Engineering, the Built Environment and Information Technology at the Nelson Mandela Metropolitan University April 2018 Supervisors: Dr R. Harris Co-Supervisor: Mr A. Roberts Industrial Mentor: Mr Eben Nyandoro IMPACT OF INTERGRATING TEEBUS HYDRO POWER ON THE UNBALANCED DISTRIBUTION MV NETWORK Lindani Mthethwa - 213486806 DECLARATION I, Lindani Mthethwa, declare that the contents of this Dissertation represent my own unaided work, and that the Dissertation has not previously been submitted for academic examination towards any qualification. Furthermore, it represents my own opinions and not necessarily those of the Nelson Mandela Metropolitan University (NMMU). Signature: Date: 15 March 2018 Student Number: 213486806 ii IMPACT OF INTERGRATING TEEBUS HYDRO POWER ON THE UNBALANCED DISTRIBUTION MV NETWORK Lindani Mthethwa - 213486806 ACKNOWLEDGEMENTS The following persons and companies are acknowledged for their valued participation that contributed to the successful completion of this project: I wish to express my sincere gratitude to Dr Raymond Harris and Mr Allen Roberts for their professional guidance and availing themselves when needed on their busy schedule. Eben Nyandoro, for sharing his in depth experience and providing an on-going mentorship. Eskom Distribution, Eastern Cape Operating Unit, Bursary Committee for the financial support needed to complete this research. My guardians and colleagues for their support and unconditional love. Simphiwe Hashe and Thobeka Maqoma, my Eskom managers for allowing me time to travel to PE for progress reporting and supporting my career. -

The Status of Traditional Horse Racing in the Eastern Cape
THR Cover FA 9/10/13 10:49 AM Page 1 The Status of Traditional Horse Racing in the Eastern Cape www.ussa.org.za www.ru.ac.za THR Intro - Chp 3 FA 9/10/13 10:40 AM Page 1 The Status of Traditional Horse Racing in the Eastern Cape ECGBB – 12/13 – RFQ – 10 Commissioned by Eastern Cape Gambling and Betting Board (ECGBB) Rhodes University, Grahamstown, Eastern Cape, was awarded incidental thereto, contemplated in the Act and to advise the a tender called for by the Eastern Cape Gambling and Betting Member of the Executive Council of the Province for Economic Board (ECGBB) (BID NUMBER: ECGBB - 12/13 RFQ-10) to Affairs and Tourism (DEAT) with regard to gambling matters undertake research which would determine the status of and to exercise certain further powers contemplated in the traditional horse racing (THR) in the Eastern Cape. Act. The ECGBB was established by section 3 of the Gambling Rhodes University, established in 1904, is located in and Betting Act, 1997 (Act No 5 of 1997, Eastern Cape, as Grahamstown in the Eastern Cape province of South Africa. amended). The mandate of the ECGBB is to oversee all Rhodes is a publicly funded University with a well established gambling and betting activities in the Province and matters research track record and a reputation for academic excellence. Rhodes University Research Team: Project Manager: Ms Jaine Roberts, Director: Research Principal Investigator: Ms Michelle Griffith Senior Researcher: Mr Craig Paterson, Doctoral Candidate in History Administrator: Ms Thumeka Mantolo, Research Officer, Research Office Eastern Cape Gambling & Betting Board: Marketing & Research Specialist: Mr Monde Duma Cover picture: People dance and sing while leading horses down to race. -

A Regional Analysis of Agricultural Price Risk in South Africa
Agrekon, Vol 33, No 3 (September 1994) Van Schalkwyk and Groenewald A REGIONAL ANALYSIS OF AGRICULTURAL PRICE RISK IN SOUTH AFRICA HD van Schalkwyk and JA Groenewald Lecturer and Professor, Department ofAgricultural Economics, Extension and Rural Development, University ofPretoria, Pretoria Regional output/input price differentials and variations were calculated to evaluate price risk in South Africa. It was found that price unstable regions are not necessarily also risky regions as regions with higher output/input price ratios can handle higher price variations better. The average value of the price index for the top ten regions is over 3 times larger than the average for the ten lowest regions. The higher the prices of inputs relative to output prices (the lower the price ratio), the smaller their application to each hectare of land, and the lower the land productivity. The regional prices appears to be a function of the interaction between differential natural and economic factors in different regions. 'n Streeksanalise van landbou prysrisiko in Suid-Afrika Regionale uitset/inset prysverskille en variasies is bereken om prys risiko in Suid-Afrika te evalueer. Daar is gevind dat prys onstabiele streke the noodwendig ook hoe risiko streke is nie, want streke met hoer uitset/inset prys verhoudings kan hoer prys variasies beter verwerk. Die gemiddelde waarde van die prys indeks vir die tien top streke is nicer as 3 keer groter as die gemiddeld vir die tien laagste streke. Hoe hoer die pryse van insette relatief tot uiset pryse(hoe lac- die prys verhouding), hoe minder is hulle aanwending per hektaar, en hoe laer die grond produktiwiteit. -
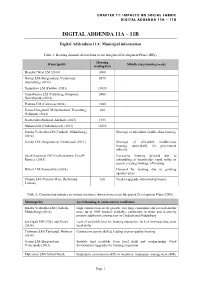
Digital Addenda 11A – 11B
CHAPTER 11: IMPACTS ON SOCIAL FABRIC DIGITAL ADDENDA 11A – 11B DIGITAL ADDENDA 11A – 11B Digital Addendum 11A: Municipal information Table 1: Housing demand, drawn from recent Integrated Development Plans (IDPs). Housing Municipality Middle class housing needs waiting lists Beaufort West LM (2014) 3000 Gariep LM (Burgersdorp, Venterstad, 4570 Steynsburg) (2013) Ngqushwa LM (Peddie) (2013) 10320 Umsobomvu LM (Colesberg, Noupoort, 2000 Norvalspont) (2014) Hantam LM (Calvinia) (2014) 1000 Karoo-Hoogland LM (Sutherland, Fraserburg, 880 Williston) (2014) Nxuba LM (Bedford, Adelaide (2013) 1923 Makana LM (Grahamstown) (2014) 16582 Inxuba Yethemba LM (Cradock, Middelburg) Shortage of affordable middle-class housing (2014) Gariep LM (Burgersdorp, Venterstad) (2011) Shortage of affordable middle-class housing, particularly for government officials Sarah Baartman DM (Grahamstown, Graaff- Increasing housing demand due to Reinet) (2015) unbundling of households; rapid influx of people creating shortage of housing Ikwezi LM (Jansenville) (2014) Demand for housing due to growing squatter areas Ubuntu LM (Victoria West, Richmond, 820 Need to upgrade old township houses Loxton) Table 2: Construction industry in various localities, drawn from recent Integrated Development Plans (IDPs). Municipality Local housing & construction conditions Inxuba Yethemba LM (Cradock, High construction sector growth; two large companies and several smaller Middelburg) (2014) ones; up to 1000 workers available; constraints in water and electricity prevent additional construction in Cradock and Middelburg Joe Gqabi DM (HQ Lady Grey) Lack of available land for housing expansion; lack of serviced sites; poor (2014) local skills Tsolwana LM (Tarkastad, Hofmeyr Contractors poorly skilled, leading to poor quality housing (2014) Gariep LM (Burgersdorp, Suitable land available. Poor local skills and workmanship. Need Venterstad) (2013) infrastructure upgrades for housing expansion Maletswai LM (Aliwal North, Inadequate construction skills in localities; inadequate management skills. -

Aliwal North / Burgersdorp /Jamestown / Steynsburg and Venterstad Notice No: 112/2019/2020 Advertisement Internal / External
ALIWAL NORTH / BURGERSDORP /JAMESTOWN / STEYNSBURG AND VENTERSTAD NOTICE NO: 112/2019/2020 ADVERTISEMENT INTERNAL / EXTERNAL The Walter Sisulu Local Municipality is a Category 2 Municipality, with its seat authority in Burgersdorp. It is situated within the Joe Gqabi District Municipal Area, in the Eastern Cape. The municipality is looking for the services of vibrant, committed, suitable, qualified and experienced individuals to fill the following position: MOTOR VEHICLES EXAMINER X 1 TASK GRADE: 10 SALARY SCALE: R 212 472.00 – R 275 820.00 PER ANNUM MINIMUM REQUIREMENTS: • Grade 12 / Matric. • Diploma in Vehicle Examiner Grade A. • Diploma Examiner of Driving license an added advantage. • Traffic Diploma is an added advantage. • Qualification in testing of leaners license will serve as an added advantage. • Qualification in examining motor vehicles. • Qualification in testing of K53 drivers licenses. • A valid Driver’s license. • Code EC driver’s license will serve as an added advantage. • Municipal experience will be a strong recommendation. • At least 2 years’ relevant experience. • Knowledge of eNatis will serve as an added advantage. • Be able to do Law enforcement when required to do so. Adverts: Managers: Assets, Financial Accounting; Traffic Officer, Examiner driver’s License Page 1 And Examiner of Vehicles • Must be able to work in any area within the jurisdiction of the municipality. • Applicants will be practically assessed. DUTIES: The duties will include but not limited to the following: • Physically examine vehicles from the public as well as vehicles brought in by law enforcement staff. • Test driving the vehicle in terms of the road traffic act to ensure that they are roadworthy • Suspending the vehicles if required • Issuing roadworthiness certificates of fitness to those vehicles that apply • Maintaining daily statistics of vehicles examined • Handling enquiries from the public regarding the roadworthiness of their vehicles to ensure that the daily statistics are recorded for future use and that the public is aware of the necessary requirement. -

Detailed First Quarter 2018/19 Progress Report
DWS 2018/19 first quarter report DETAILED FIRST QUARTER 2018/19 PROGRESS REPORT No milestone for the quarter Over achieved Achieved Partially achieved Not achieved APP (Annual Performance Plan) ADD ( Additional ) Indicators & target as per 2018/19 Annual Performance Plan (APP) Quarter 1 Analysis of perfomance PPI No Performance Indicators Target for 2018/19 as per Annual Progress Description of Actual Reason for Deviation Planned Action Analysis Performance Plan (APP) Achievement Quarter 1 Target as per Disaggregated Quarter 1 output – preliminary Quarter 1 Actual Deviation (Actual) Deviation (%) APP targets per province Achievement / CMA Programe1: Administration Departmental Management sub-programme 4.3.1 Percentage compliance with Annual 100% compliance n/a No milestone No milestone n/a n/a No milestone n/a n/a No milestone MPAT standards at the minimum targeted level 3 Corporate Services sub-programme 4.3.4 Percentage vacancy rate for ≤10% ≤10% n/a 121% 121% Over by 31% Over by 31% 121% filled over establishment. (i.e 711 The Department has a total establishment n/a Over achieved engineers and scientists posts filled out of 586 permanent posts.. of 586 permanent posts on Persal. The Filled over establishment. (i.e 711 posts filled out of 586 Filled over establishment. (i.e 711 posts Formula: Formula: Department has filled 711 posts. permanent posts. filled out of 586 permanent posts. 121% - 90%=31% 711/586*100=121% Formula: 711/586*100=121% Formula: 711/586*100=121% 4..4.2 Number of learners that 2640 No milestone n/a No milestone -

Proposed Main Seat / Sub District Within the Proposed Magisterial
!C ñ!.C!^ !C$ ^!C ^ ^ !C !C !C !C !C ^ !C !C ^ !C^ !C !C !C !C !C ^ !C ñ !C !C !C !C !C !C ^ !C !C !C ^ $ !C ^ !C !C !C !C !C !C ^ ^!C !C ñ !C !C !C !C !C !C !C !C !C !C !C !. !C ^ !C ^ !C ñ !C !C !C $ !C !C!C ^ !C !C ^ !C !C ñ !C !C !C !.!C^ ñ!C ñ !C !C !C ^ !C !C ^ ^ !C ^ !C !C !C !C !C !C !C !C ^ !C !C !C ñ !C !C ^ !C ñ !C !C !C ñ !C !C !C !C !C !C !C !C !C !C !C ñ !C ^ !C ^ !C !C !C ñ!C ^!C !. ^ !C !C ñ!C ^ !C !C ^ $ ^$!C ^ !C !C !C !C !C !C !C !C !C !C !C !C !. !C !.^ $ !C !C ñ !C !C ^ !C !C !C $ ^ !C !C $!C !C $ !C !C ñ !. !C !C !C!C !C ñ!C!. ^ ^ ^ !C $!. !C^ !C !C !C !C !C !C !C !C !C !C !C !C !C!C !. !C !C !C !C ^ !C !C !.!C !C !C ñ !C !C^ñ !C !C ñ !C !C !C!.^ !C !C !C !C !C ^!Cñ ^$ ^ñ!C !C ñ!C!.^ !C !C !. ^ !C ^ñ !. !C $^ ñ^!C ^ !C ^ ^ ñ ^ !C !C !C !C !C !C ^ !C !C !C !C !C !C !C !C !C !. !C ^ !C $ !. ñ!C !C !C ^ ñ!C.^ !C !C !C !C !C !C !C !C ^!.$C !. !. !C ^ !C !C!. !C ^ !C !C^ !C !C !C ñ !. !C $^ !C !C !C !C !C !C !. -

Local Economic Development Strategy Review Situational Analysis 2014-2019
LOCAL ECONOMIC DEVELOPMENT STRATEGY REVIEW SITUATIONAL ANALYSIS 2014-2019 Situational Analysis Report Contents 1 Introduction ................................................................................................................. 1 1.1 Project Area ........................................................................................................... 1 1.2 Objectives Of The Reviewed LED Strategy ............................................................ 2 1.3 Methodology .......................................................................................................... 2 1.4 Report Structure ..................................................................................................... 3 1.4.1 Structure of the Document.............................................................................................. 3 1.5 Principles ............................................................................................................... 5 1.5.1 Sustainable Development............................................................................................... 5 1.5.2 Local Economic Development ........................................................................................ 6 2 Policy Framework ........................................................................................................ 8 2.1 International Perspective ........................................................................................ 8 2.1.1 Millennium Development Goals .....................................................................................