Texas Root Rot in Pecans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Texas Root Rot Compiled by Chris Anderson (NSW DPI)
Fact sheet Texas root rot Compiled by Chris Anderson (NSW DPI) What is Texas root rot? Texas root rot, caused by the fungus Phymatotrichopsis omnivora, is one of the most destructive fungal plant diseases. It is a soil-borne fungus that attacks the roots of susceptible plants. It causes sudden wilt and death of affected plants, usually during the warmer months. Texas root rot affects over 2000 species of plants. It is an important disease of cotton as well as alfalfa, Chris Anderson, I&I NSW grapes, fruit trees, and many ornamentals. Cotton plants infected with Texas root rot fungus, which have developed yellow, wilting leaves What does it look like? Plants initially wilt during hot weather as the rotted roots are unable to take up enough water and the stem may become girdled at soil level. Soon after this the plant will die. The dead leaves usually remain attached to the plant. At this stage, the roots are dead and their surface is covered with a network of white to tan fungal strands. Affected areas expand to form circular patches of dead plants. What can it be confused with? Chris Anderson, I&I NSW Tractor driver’s view of severely damaged cotton field Sudden wilt, Fusarium wilt and lightning strike. What should I look for? In the field, look for patches of dead and dying plants (often with the dead leaves still attached). Patches may expand in a circular pattern during warm weather as the fungus spreads through the soil from plant to plant. Dead plants should be pulled up and examined for the presence of white to tan fungal strands on the roots and girdling of the stem at ground level. -

Cotton Root Rot (Phymatotrichopsis Root Rot) and Its Management
PLPA-FC010-2016 COTTON ROOT ROT (PHYMATOTRICHOPSIS ROOT ROT) AND ITS MANAGEMENT Phymatotrichopsis root rot (also known as cotton root rot, Phymatotrichum root rot, Texas root rot and Ozonium root There is white to brown fungal growth on the surface of rot) is a major fungal disease of cotton occurring within large main roots near the lower stem, consisting of strands areas of Texas and Arizona, causing annual losses in Texas and a loose, cottony growth just below the soil surface alone of up to $29 million. The causal fungus is soilborne (Fig. 3). and has a host range of more than 1800 dicot plants. This disease only occurs in the southwestern United States and several northern states of Mexico. There has been no expansion of geographic range of the disease within North America. Diagnosis and Impact The disease develops late in the spring or early summer, as soil temperatures approach 82°F. About a day before the onset of visible symptoms , the leaves of infected plants feel noticeably hotter than surrounding, non-infected plants. The Fig. 3 Growth of cotton root rot fungus on root and first visible symptom is wilting (Fig. 1), which becomes base of stem. permanent by the third day, Wilt is usually seen when plants are flowering, sometimes earlier in the season, but not when plants are seedlings. A large number of plants may wilt simultaneously, but even within an affected area, wilting among plants is not simultaneous, sometimes occurring weeks apart. It is also possible to see non- symptomatic plants surrounded by diseased plants. -

Phymatotrichum Omni- Vorum
PERSISTENT STRANDS OF THE COTTON ROOT-ROT FUNGUS IN TEXAS' By HoMHR C. MCNAMARA, associate agronomist^ and R. E. WESTER and K. C. GuNN, assistant scientific aids, Division of Cotton and Other Fiber Crops and Diseases, Bureau of Plant Industry, United States Department of Agriculture INTRODUCTION The ability of the cotton root-rot fungus (Phymatotrichum omni- vorum (Shear) Dug.) (2) ^ to remain in the soil in a viable and infec- tious condition for a period of years, even when the fields are planted to nonsusceptible crops or kept in clean fallow, has been a problem of much concern to all mvestigators of this disease. Further observa- tions on the ability of this fungus to perpetuate itself in the soil for long periods were made possible during the summer of 1932, when plots that had been in clean fallow for several years or planted to nonsusceptible crops were returned to cotton. Even after a 5-year clean fallow, several centers of infection appeared in a half-acre plot during July and August. In the planning of experimental work on the control of cotton root rot, the nature and stage of the organism that is being dealt with must be considered. Clean fallows covering from 1 to 5 years and the use of nonsusceptible crops on infested areas have furnished con- ditions under which it has been possible to study the fungus in the soil on both cropped and uncropped plots. The colloidal nature of the Wilson and Houston clay soils presented special opportunities for studying the fungus in its natural state of growth. -

Septal Characteristics and General Ultrastructure of Phymatotrichum Omnivorum (Shear) Duggar
Septal characteristics and general ultrastructure of Phymatotrichum omnivorum (Shear) Duggar Item Type text; Thesis-Reproduction (electronic) Authors Dong, Sophie, 1952- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 28/09/2021 18:41:54 Link to Item http://hdl.handle.net/10150/348212 SEPTAL CHARACTERISTICS AND GENERAL ULTRASTRUCTURE OF PRYMATOTRICHUM OMNIVORUM (SHEAR) DUGGAR by - Sophie Dong A Thesis Submitted to the Faculty of the DEPARTMENT OF PLANT PATHOLOGY In Partial Fulfillment of the Requirements For the Degree of MASTER OF SCIENCE In the Graduate College THE UNIVERSITY OF ARIZONA 1 9 7 7 STATEMENT BY AUTHOR This thesis has. been submitted in partial fulfillment of re quirements for an advanced degree at The University of Arizona and is deposited in the University Library to be made available to borrowers under rules of the Library, Brief quotations from this thesis are allowable without special permission, provided that accurate acknowledgment of source is made. Requests for permission for extended quotation from or reproduction of this manuscript in whole or in part may be granted by the head of the major department or the Dean of the Graduate College when in his judg ment the proposed use of the material is in the interests of scholar ship, In all other instances, however, permission must be obtained from the author. SIGNED s_ I?- APPROVED BY THESIS DIRECTOR This thesis has been approved on the date shown below: g / W 7 7 H. -

Texas Root Rot FS Viticulture
Fact sheet Texas root rot What is it? Texas root rot, caused by the fungus Phymatotrichum omnivora, is one of the most destructive fungal plant diseases. It causes sudden wilt and death of affected plants, usually during the warmer months. It is a soil-borne fungus that attacks the roots of susceptible plants. Texas root rot affects over 2000 species of plants including fruit and nut trees, oleander and roses. This is an important disease of cotton, alfalfa, grapes, fruit trees, and many ornamentals. All varieties of grapes are susceptible, but Vitis vinifera varieties seem to be exceptionally susceptible. Department of Plant Pathology, University of Arizona Plant Pathology,Department of of University © What do I look for? Grapevines affected by Texas root rot As the damaged roots are unable to take up enough water to maintain the plant in warm weather, symptoms only become obvious during the summer. The leaves wilt and the plant dies. University of Arizona of University The dead leaves usually remain attached to the plant. At this stage, the roots are dead and their surface is covered with a network of tan fungal strands. Affected areas expand to form circular areas of dead plants. Department of Plant Pathology,Department of © Mycelial strands of Texas root rot How does it spread? Locally, the disease spreads from infected to healthy roots via fungal strands which grow through the soil. Long-distance spread most often occurs through the movement of soil or roots of infected host plants. The fungus does not spread readily from one location to another. Bugwood.org Where is it found? S.D. -
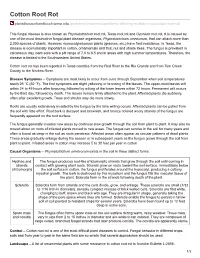
Cotton Root Rot
Cotton Root Rot plantdiseasehandbook.tamu.edu /problems-treatments/problems-affecting-multiple-crops/cotton-root-rot/ This fungal disease is also known as Phymatotrichum root rot, Texas root rot and Ozonium root rot. It is caused by one of the most destructive fungal plant disease organisms, Phymatotrichum omnivorum, that can attack more than 2,000 species of plants. However, monocotyledonous plants (grasses, etc.) have field resistance. In Texas, the disease is economically important in cotton, ornamentals and fruit, nut and shade trees. The fungus is prevalent in calcareous clay loam soils with a pH range of 7.0 to 8.5 and in areas with high summer temperatures. Therefore, the disease is limited to the Southwestern United States. Cotton root rot has been reported in Texas counties from the Red River to the Rio Grande and from Tom Green County to the Neches River. Disease Symptoms – Symptoms are most likely to occur from June through September when soil temperatures reach 28 °C (82 °F). The first symptoms are slight yellowing or bronzing of the leaves. The upper-most leaves wilt within 24 to 48 hours after bronzing, followed by wilting of the lower leaves within 72 hours. Permanent wilt occurs by the third day, followed by death. The leaves remain firmly attached to the plant. Affected plants die suddenly, often after excellent growth. Trees and shrubs may die more slowly. Roots are usually extensively invaded by the fungus by the time wilting occurs. Affected plants can be pulled from the soil with little effort. Root bark is decayed and brownish, and bronze colored wooly strands of the fungus are frequently apparent on the root surface. -

Phymatotrichum Root Rot
Oklahoma Cooperative Extension Service EPP-7621 Phymatotrichum Root Rot John P. Damicone State Extension Plant Pathology Specialist Oklahoma Cooperative Extension Fact Sheets are also available on our website at: Phymatotrichum root rot, also known as cotton root rot http://osufacts.okstate.edu and Texas root rot, is caused by the fungus Phymatotrichopsis ominvorum. The fungus attacks more than 2,000 species of broadleaf plants, but does not affect monocots (grasses). The disease is economically important to cotton, alfalfa, peanut, sclerotia. Sclerotia have been recovered from soil at depths ornamental shrubs, and fruit, nut and shade trees. of up to 8 feet. In the summer, mycelium from germinating The fungus is capable of persisting for a long period sclerotia or from fungal fragments overwintering on roots of of time in the soil. It is most destructive in soils that are of perennial plants infects nearby roots. The fungus enters the limestone origin (calcareous), highly alkaline (high pH), and roots of infected plants, decaying the bark and penetrating the are exposed to high summer temperatures. Therefore, soil woody portions of the root. Nutrients derived from infected type and cold temperatures limit the geographic distribution plants support the growth of the fungus through the soil to of this disease to areas in the southwestern and south cen- infect new plants. The function of spores produced on spore tral United States. In Oklahoma, Phymatotrichum root rot is mats in the disease cycle is unknown. mostly restricted to the southern tier of counties in the Red The fungus appears to be indigenous to certain fields and River Valley. -

Euonymus Fortunei
Euonymus fortunei INTRODUCTORY DISTRIBUTION AND OCCURRENCE BOTANICAL AND ECOLOGICAL CHARACTERISTICS FIRE EFFECTS AND MANAGEMENT MANAGEMENT CONSIDERATIONS APPENDIX: FIRE REGIME TABLE REFERENCES INTRODUCTORY AUTHORSHIP AND CITATION FEIS ABBREVIATION NRCS PLANT CODE COMMON NAMES TAXONOMY SYNONYMS LIFE FORM FEDERAL LEGAL STATUS OTHER STATUS Photo by Keith Langdon, National Park Service, Bugwood.org AUTHORSHIP AND CITATION: Zouhar, Kris. 2009. Euonymus fortunei. In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Available: http://www.fs.fed.us/database/feis/ [ 2010, January 25]. FEIS ABBREVIATION: EUOFOR NRCS PLANT CODE [69]: EUFO5 EUFOF EUFOR2 COMMON NAMES: wintercreeper Chinese spindle-tree climbing euonymus TAXONOMY: The scientific name of wintercreeper is Euonymus fortunei (Turcz.) Hand.-Maz. (Celastraceae) [3,18,22,33,42,46,73,76]. Although not distinguished in local floras, Kartesz [33] recognizes 2 varieties in North America: Euonymus fortunei (Turcz.) Hand.-Maz. var. fortunei Euonymus fortunei (Turcz.) Hand.-Maz. var. radicans (Sieb. ex Miq.) Rehd. A review by Dirr [17] describes another variety in the United States, E. f. var. coloratus, which he says is often listed as the cultivar 'Coloratus'. Dirr [17] describes 51 cultivars of E. fortunei. A review by Blakelock [2] describes several varieties and forms of E. fortunei, occurring mostly in Asia or as cultivars, distinguished primarily by leaf characteristics. The Flora of China states "Numerous taxa have been named within the E. fortunei complex but many of these refer to cultivated plants and are best treated as cultivars" [76]. In this review, E. fortunei is referred to as "wintercreeper", varieties are referred to by scientific name, and cultivars, when identified as such in the literature, are referred to by cultivar name in single quotation marks (e.g., 'Emerald and Gold'). -

Benjamin Minge Duggar
NATIONAL ACADEMY OF SCIENCES BEN J AMIN MINGE D UGGAR 1872—1956 A Biographical Memoir by J . C . W A L K E R Any opinions expressed in this memoir are those of the author(s) and do not necessarily reflect the views of the National Academy of Sciences. Biographical Memoir COPYRIGHT 1958 NATIONAL ACADEMY OF SCIENCES WASHINGTON D.C. BENJAMIN MINGE DUGGAR September i, 1872—September io, 1956 BY J. C. WALKER HEN BENJAMIN MINGE DUGGAR passed away on September 10, W1956, at New Haven, Connecticut, he was well along in the seventh decade of his scientific activity. Few scientists have con- tributed over so long a period in so many areas of biological science. His mother, Margaret Louisa (Minge) Duggar, was the daughter of a plantation owner in eastern Virginia. His father, Reuben Henry Duggar, was a native of central Alabama where he practiced medi- cine, primarily in the rural areas. He served a term of duty in the Confederate Army during the Civil War. Benjamin Minge Duggar, the fourth of six sons, was born on September 1, 1872, at Gallion, Alabama. His secondary education was gained in the local school and under private tutors. That he was a precocious youth is shown by the fact that he entered the Univer- sity of Alabama within a few days of his fifteenth birthday. After two years at that institution he transferred to the Mississippi Agri- cultural and Mechanical College (now Mississippi State College), near Starkville, Mississippi. This transfer would lead one to surmise that he had developed an early interest in agricultural science. -

Pest Categorisation of Phymatotrichopsis
SCIENTIFIC OPINION ADOPTED: 31 January 2019 doi: 10.2903/j.efsa.2019.5619 Pest categorisation of Phymatotrichopsis omnivora EFSA Panel on Plant Health (PLH), Claude Bragard, Katharina Dehnen-Schmutz, Francesco Di Serio, Paolo Gonthier, Marie-Agnes Jacques, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas-Cortes, Stephen Parnell, Roel Potting, Philippe Lucien Reignault, Hans-Hermann Thulke, Wopke Van der Werf, Jonathan Yuen, Lucia Zappala, Michael Jeger, Irene Vloutoglou, Bernard Bottex and Antonio Vicent Civera Abstract The Panel on Plant Health performed a pest categorisation of Phymatotrichopsis omnivora, the causal agent of Phymatotrichum root rot of more than 2,000 dicotyledonous plant species, for the EU. The pest is listed as Trechispora brinkmannii in Annex IAI of Directive 2000/29/EC. P. omnivora is a well- defined fungal species and reliable methods exist for its detection and identification. It is present in south-western USA, northern Mexico, Libya and Venezuela. The pest is not known to occur in the EU. P. omnivora has an extremely wide host range; quantitative impacts have been documented for Gossypium spp. (cotton), Medicago sativa (alfalfa), Malus domestica (apple), Prunus persica (peach) and Vitis vinifera (grapevine) as the major cultivated hosts. All major hosts and pathways of entry of the pest into the EU are currently regulated, except for soil and growing media attached or associated with plants originating in Libya. Host availability and climate and edaphic matching suggest that P. omnivora could establish in parts of the EU and further spread mainly by human-assisted means. The pest infects the roots causing wilting and death of its host plants. -

Plants for the Desert Southwest Our Vision: to Introduce, Provide and Popularize Desert-Adapted Plants for Southwestern Landscapes
Plants for the Desert Southwest Our Vision: To introduce, provide and popularize desert-adapted plants for Southwestern landscapes. As we celebrate 40 years in business, we are excited to share the second edition of our plant catalog with you! We have had tremendous feedback regarding the first edition. We’d like to thank our many plant friends who have reviewed the first copy and provided input. This catalog contains information on nearly 400 taxa of trees, shrubs, ornamental grasses, accents, flowering perennials, groundcovers and vines.The most current botanical names were used, and since we know old habits die hard, we have included a “cheat sheet” to help you cross-reference old and new botanical names.The hardiness information listed is based on the best knowledge currently available. Regional environments vary widely and microclimates will have a great effect on plant hardiness. Please bear in mind that this data is provided as a general guide to help you with plant selection.The information presented in this catalog, along with a host of other information and photographs, is available on-line at: www.mswn.com. Many thanks to all of our customers and friends who have supported and encouraged us through the years! We hope to see you soon! Contents Contact Information 1–58 Plant List Mailing Address: P.O. Box 2500 Litchfield Park,AZ 85340-2500 59 Notes Page Nursery Address: 60 Map to Nursery 10020 W. Glendale Avenue Glendale,AZ 85307 60 Delivery, Ordering & Payment Information Phone: (623) 247-8509 or (800) 840-8509 60 Custom Growing Fax: (623) 247-6354 61 Cross Reference of Botanical Names www.mswn.com BOTANICAL NAME COMMON NAME ELEVATION SIZE WATER FLOWER MIN USDA LOW MIDDLE HIGH (H x W) USE COLOR TEMP ZONE Abutilon palmeri Indian Mallow X 5 x 5 Low Apricot 25 9 This herbaceous shrub has soft, velvety, heart-shaped leaves. -

Diseases of Cotton X
INTERIM REPORT 2010 to 2012 Diseases of Cotton X Karen Kirkby Peter Lonergan Bethany Cooper Locked Bag 1000, Narrabri, NSW, 2390, Australia 1 Contents Contents .........................................................................................................................2 Contact Details ..............................................................................................................3 Intellectual Property developed within the projects. .....................................................4 Acknowledgements.........................................................................................................4 Background....................................................................................................................5 Objectives and extent to which they have been achieved ..............................................7 Chapter 1 - Disease Surveys..........................................................................................9 Introduction: ................................................................................................................9 Method and Materials:................................................................................................16 Results & Discussion:..................................................................................................17 Conclusions:...............................................................................................................32 Chapter 2 - Seed Treatments .......................................................................................34