The Influence of Selected Food Ingredients on the Release of Aroma Compounds from Β-Cyclodextrin in Aqueous Solutions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC
Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC R E S E A R C H A N D D E V E L O P M E N T EPA/600/R-06/105 September 2006 Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC By S. H. Hilal Ecosystems Research Division National Exposure Research Laboratory Athens, Georgia U.S. Environmental Protection Agency Office of Research and Development Washington, DC 20460 NOTICE The information in this document has been funded by the United States Environmental Protection Agency. It has been subjected to the Agency's peer and administrative review, and has been approved for publication. Mention of trade names of commercial products does not constitute endorsement or recommendation for use. ii ABSTRACT SPARC (SPARC Performs Automated Reasoning in Chemistry) chemical reactivity models were extended to calculate hydrolysis rate constants for carboxylic acid ester and phosphate ester compounds in aqueous non- aqueous and systems strictly from molecular structure. The energy differences between the initial state and the transition state for a molecule of interest are factored into internal and external mechanistic perturbation components. The internal perturbations quantify the interactions of the appended perturber (P) with the reaction center (C). These internal perturbations are factored into SPARC’s mechanistic components of electrostatic and resonance effects. External perturbations quantify the solute-solvent interactions (solvation energy) and are factored into H-bonding, field stabilization and steric effects. These models have been tested using 1471 reliable measured base, acid and general base-catalyzed carboxylic acid ester hydrolysis rate constants in water and in mixed solvent systems at different temperatures. -

Assessment of Free and Immobilized Kefir Culture in Simultaneous
LWT - Food Science and Technology 76 (2017) 67e78 Contents lists available at ScienceDirect LWT - Food Science and Technology journal homepage: www.elsevier.com/locate/lwt Assessment of free and immobilized kefir culture in simultaneous alcoholic and malolactic cider fermentations Anastasios Nikolaou a, Alex Galanis a, Maria Kanellaki b, Chrysoula Tassou c, * Konstantoula Akrida-Demertzi d, Yiannis Kourkoutas a, a Laboratory of Applied Microbiology and Biotechnology, Department of Molecular Biology & Genetics, Democritus University of Thrace, Alexandroupolis, GR-68100, Greece b Food Biotechnology Group, Section of Analytical Environmental and Applied Chemistry, Department of Chemistry, University of Patras, Patras, GR-26500, Greece c Institute of Technology of Agricultural Products, Hellenic Agricultural Organization DEMETER, 1 S. Venizelou Str, Lykovrissi, Athens, GR-14123, Greece d Laboratory of Food Chemistry and Technology, Department of Chemistry, University of Ioannina, Dourouti, Ioannina, GR-45110, Greece article info abstract Article history: The aim of the present study was to assess application of free or immobilized kefir culture on apple Received 30 March 2016 pieces and delignified cellulosic material (DCM) in simultaneous alcoholic and malolactic cider fer- Received in revised form mentations at a wide temperature range (5e45 C). Repeated batch fermentations were continued for 12 October 2016 higher than 7 months, showing a high operational stability of the systems and were completed in less Accepted 13 October 2016 than 24 h with immobilized cells on DCM at 37 C. Malic acid conversion up to 71.5% and ethanol Available online 15 October 2016 productivity values up to 56.9 g/(Ld) were recorded, which could be adopted by the industrial sector. -

Comprehensive Mapping of Volatile Organic Compounds in Fruits
International PhD Program in Biomolecular Sciences XXVII Cycle Comprehensive Mapping of Volatile Organic Compounds in Fruits Tutor Dr. Fulvio Mattivi Department of Food Quality and Nutrition, Fondazione Edmund Mach Advisor Prof. Vladimir Shulaev Department of Biological Sciences, University of North Texas Ph.D. Thesis of Manoj Shahaji Ghaste Department of Food Quality and Nutrition Fondazione Edmund Mach 2013-2014 This thesis is lovingly dedicated to my Mother. Her support, encouragement, belief and constant love have sustained me throughout my life. Declaration I, Manoj Shahaji Ghaste confirm that this is my own work and the use of all material from other sources has been properly and fully acknowledged. Thesis abstract Volatile organic compounds (VOCs) are the key aroma producers in fruits and sensory quality of fruits is widely determined by qualitative and quantitative composition of VOCs. The aroma of grape is a complex of hundreds of VOCs belonging to different chemical classes like alcohols, esters, acids, terpenes, aldehydes, furanones, pyrazines, isoprenoids and many more. VOCs play important role as they determine the flavor of grapes and wine made from it. The objective of this thesis is to study of VOCs through development of different mass spectrometry based analytical methodologies and its applications for the comprehensive investigation and construction of database of the VOCs in grapes. First part of the study was dedicated to generation of a database of grape VOCs through the screening of multiple grape varieties (n=124) representing different species, color and origin. The experiment was carried out using headspace solid-phase microextraction (HS-SPME) and gas chromatography mass spectrometry (GC-MS) based approach and according to metabolomics protocols. -

Comprehensive Overview of Common E-Liquid Ingredients and How They Can Be Used to Predict an E-Liquid's Flavour Category
Original research Tob Control: first published as 10.1136/tobaccocontrol-2019-055447 on 10 February 2020. Downloaded from Comprehensive overview of common e- liquid ingredients and how they can be used to predict an e- liquid’s flavour category Erna J Z Krüsemann ,1,2 Anne Havermans ,1 Jeroen L A Pennings,1 Kees de Graaf,2 Sanne Boesveldt,2 Reinskje Talhout1 10–14 ► Additional material is ABSTRact cigarette smoking. In line with this, the use and published online only. To view Objectives Flavours increase e- cigarette attractiveness marketing of e- liquid flavours that are appealing to please visit the journal online (http:// dx. doi. org/ 10. 1136/ and use and thereby exposure to potentially toxic smokers may contribute to public health benefits. tobaccocontrol- 2019- 055447). ingredients. An overview of e- liquid ingredients is needed However, flavours may also stimulate vaping among to select target ingredients for chemical analytical and non- users, in particular young people.15–17 This is 1 Centre for Health toxicological research and for regulatory approaches concerning, as e- cigarettes are not safe10 18 19 That Protection, Rijksinstituut voor aimed at reducing e- cigarette attractiveness. Using is, chemicals in e- cigarette emissions (eg, tobacco- Volksgezondheid en Milieu, Bilthoven, The Netherlands information from e- cigarette manufacturers, we aim to specific nitrosamines, metals, aldehydes and other 2Division of Human Nutrition identify the flavouring ingredients most frequently added flavourings) can be toxic and thus harmful to and Health, Wageningen to e- liquids on the Dutch market. Additionally, we used consumers’ health.20–22 In addition, e- cigarettes University, Wageningen, The flavouring compositions to automatically classify e-liquids may facilitate smoking initiation among never- Netherlands into flavour categories, thereby generating an overview smokers.23 As a consequence, e- liquid flavours are that can facilitate market surveillance. -

Novel Analysis on Aroma Compounds of Wine, Vinegar and Derived Products
Novel Analysis on Aroma Compounds of Wine, Vinegar and Derived Products Edited by Enrique Durán-Guerrero and Remedios Castro-Mejías Printed Edition of the Special Issue Published in Foods www.mdpi.com/journal/foods Novel Analysis on Aroma Compounds of Wine, Vinegar and Derived Products Novel Analysis on Aroma Compounds of Wine, Vinegar and Derived Products Editors Enrique Dur´an-Guerrero Remedios Castro-Mej´ıas MDPI Basel Beijing Wuhan Barcelona Belgrade Manchester Tokyo Cluj Tianjin • • • • • • • • • Editors Enrique Duran-Guerrero´ Remedios Castro-Mej´ıas University of Cadiz´ University of Cadiz´ Spain Spain Editorial Office MDPI St. Alban-Anlage 66 4052 Basel, Switzerland This is a reprint of articles from the Special Issue published online in the open access journal Foods (ISSN 2304-8158) (available at: https://www.mdpi.com/journal/foods/special issues/Wine Aroma). For citation purposes, cite each article independently as indicated on the article page online and as indicated below: LastName, A.A.; LastName, B.B.; LastName, C.C. Article Title. Journal Name Year, Volume Number, Page Range. ISBN 978-3-0365-0000-0 (Hbk) ISBN 978-3-0365-0000-0 (PDF) © 2021 by the authors. Articles in this book are Open Access and distributed under the Creative Commons Attribution (CC BY) license, which allows users to download, copy and build upon published articles, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. The book as a whole is distributed by MDPI under the terms and conditions of the Creative Commons license CC BY-NC-ND. -

Evaluation of Perceptual Interactions Between Ester Aroma Components in Langjiu by GC-MS, GC-O, Sensory Analysis, and Vector Model
foods Article Evaluation of Perceptual Interactions between Ester Aroma Components in Langjiu by GC-MS, GC-O, Sensory Analysis, and Vector Model Yunwei Niu 1, Ying Liu 1 and Zuobing Xiao 1,2,* 1 School of Perfume and Aroma Technology, Shanghai Institute of Technology, No.100 Haiquan Road, Shanghai 201418, China; [email protected] (Y.N.); [email protected] (Y.L.) 2 Beijing Advanced Innovation Center for Food Nutrition and Human Health, No. 11/33, Fucheng Road, Haidian District, Beijing 100048, China * Correspondence: [email protected]; Tel.: +86-21-60873511 Received: 3 January 2020; Accepted: 11 February 2020; Published: 13 February 2020 Abstract: The volatile compounds of three Langjiu (“Honghualangshi, HHL”, “Zhenpinlang, ZPL”, and “Langpailangjiu, LPLJ”) were studied by gas chromatography-olfactometry (GC-O) and gas chromatography-mass spectrometry (GC-MS). The results showed that a total of 31, 30, and 30 ester compounds making a contribution to aroma were present in the HHL, ZPL, and LPLJ samples, respectively. From these esters, 16 compounds were identified as important odour substances, and their odour activity values (OAVs) were greater than 1. The key ester components were selected as: ethyl acetate, ethyl 2-methylbutyrate, ethyl 3-methyl butyrate, ethyl hexanoate, and ethyl phenylacetate by aroma extract dilution analysis (AEDA), odour activity value (OAV), and omission testing. Five esters were studied for perceptual interactions while using Feller’s additive model, OAV, and a vector model. Among these mixtures, they all have an enhancing or synergistic effect. Among these mixtures, one mixture presented an additive effect and nine mixtures showed a synergistic effect. -

Supplemental Material
Haddad Supp pp 1 Supplementary materials for: Global features of neural activity in the olfactory system form a parallel code that predicts olfactory behavior and perception Rafi Haddad1,2#, Tali Weiss1, Rehan Khan1σ, Boaz Nadler2, Nathalie Mandairon3, 3 1* 1*# Moustafa Bensafi , Elad Schneidman and Noam Sobel . Section 1: PCA analysis We provide a step by step example of how we conducted the PCA analysis. Assume we have 8 odors, each located on the vertexes of a 3 dimensional unit cube. Each odor can thus be represented by the exact binary code of the numbers 0 to 7. We can represent these odors in the following binary code matrix: Odor ID Pattern code: 1 0 0 0 2 0 0 1 3 0 1 0 4 0 1 1 5 1 0 0 6 1 0 1 7 1 1 0 8 1 1 1 1 Haddad Supp pp 2 Using any available mathematical tool (we used Matlab 'princomp' method) we can calculate the principle components scores of this matrix. In this case the values of the PC1 scores are: [1 0 0]. The PC1 projection value of each row is the projection of each row by the PC1 weight vector. For example the PC1 value of the first row is [0,0,0]X[1,0,0] = 0X1 + 0X0 + 0X0 = 0. (this is a vector multiplication). Thus the PC1 value of each row of this matrix is: 0,0,0,0,1,1,1,1. Note that usually the PC1 is calculated on a centered matrix (the columns of the matrix have zero mean) and thus the PC1 value might be shifted by some value. -

Differential Analysis of Proteins Involved in Ester Metabolism in Two
microorganisms Article Differential Analysis of Proteins Involved in Ester Metabolism in two Saccharomyces cerevisiae Strains during the Second Fermentation in Sparkling Wine Elaboration Maria del Carmen González-Jiménez 1, Jaime Moreno-García 1 , Teresa García-Martínez 1,* , Juan José Moreno 2 , Anna Puig-Pujol 3, Fina Capdevilla 3 and Juan Carlos Mauricio 1 1 Department of Microbiology, University of Cordoba, 14014 Cordoba, Spain; [email protected] (M.d.C.G.-J.); [email protected] (J.M.-G.); [email protected] (J.C.M.) 2 Department of Agricultural Chemistry, University of Cordoba, 14014 Cordoba, Spain; [email protected] 3 Department of Enological Research, Institute of Agrifood Research and Technology-Catalan Institute of Vine and wine (IRTA-INCAVI), 08720 Barcelona, Spain; [email protected] (A.P.-P.); [email protected] (F.C.) * Correspondence: [email protected]; Tel.: +34 957218640; Fax: +34 957218650 Received: 29 January 2020; Accepted: 11 March 2020; Published: 13 March 2020 Abstract: The aromatic metabolites derived from yeast metabolism determine the characteristics of aroma and taste in wines, so they are considered of great industrial interest. Volatile esters represent the most important group and therefore, their presence is extremely important for the flavor profile of the wine. In this work, we use and compare two Saccharomyces cerevisiae yeast strains: P29, typical of sparkling wines resulting of second fermentation in a closed bottle; G1, a flor yeast responsible for the biological aging of Sherry wines. We aimed to analyze and compare the effect of endogenous CO2 overpressure on esters metabolism with the proteins related in these yeast strains, to understand the yeast fermentation process in sparkling wines. -
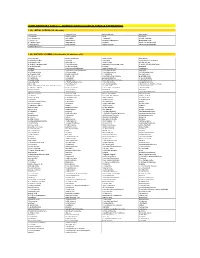
Supplementary Table 1 - Odorant Panels Used in Various Experiments
SUPPLEMENTARY TABLE 1 - ODORANT PANELS USED IN VARIOUS EXPERIMENTS 1 (A). INITIAL SCREEN (25 odorants) (-)-carvone 2-pentyl furan benzaldehyde isoeugenol 1,4-cineole 4-allyl anisole citral limonene 1-methyl pyrrole allyl sulfide eucalyptol methyl salicylate 1-octen-3-ol alpha-ionone gamma-heptalactone propionaldehyde 2,3-butanedione alpha-pinene guaiacol trans-3-hexenoic acid 2-ethyl phenol amyl acetate isobutyric acid trans-cinnamaldehyde 2-methyl pyrazine 1 (B). MIXTURE SCREEN (348 odorants, 58 mixtures of 6) hexyl acetate propyl propionate ethyl caprate isoeugenol 6-methylquinoline geraniol 1-nonanol tetrahydrofurfuryl alcohol isobutyraldehyde alpha-pinene ethyl acrylate phenyl actetate 2-methyl-1-propane thiol 1-methyl pyrrole alpha amyl cinnamaldehyde 3-acetyl-2,5-dimethyl furan 4-methyl anisole methyl myristate decanol bacdanol nonanal 2-methyl butyraldehyde trans-3-hexen-1-ol floralozone pyridine 2-tert-butyl cyclohexanone 1,4-butane dithiol veratrole ethyl heptanoate (+)-rose oxide 2-methyl pyrazine (+/-)-4-heptanolide pelargonic acid heptyl isobutyrate (-)-2-butanol acetophenone cis-3-hexen-1-ol ethyl acetate 3-methylcyclopentanone p-anisaldehyde 3-heptanone eucalyptol pentyl propionate 2-acetyl thiazole isoamyl amine 3-decanone bis-(methylthio) methane 2-phenyl ethanol valeraldehyde 2,5-dimethyl pyrazine 2-methyl anisole styralyl propionate phenetole (+/-)-citronellal 2-methoxy pyrazine ethyl ortho methoxy benzoate alpha-amyl cinnamaldehyde dimethyl acetal 2-nonanone acetaldehyde 2-propenyl phenol 1,3-butane dithiol diethyl sebacate -

CAS # IUPAC Name/Chemical Name
CAS # IUPAC Name/Chemical Name/Essenal Oil CAS, Common Name 57-10-3 Hexadecanoic acid Palmi&c acid, natural 57-55-6 Propane-1,2-diol Propylene glycol 59-02-9 (2R)-2,5,7,8-Tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydrochromen-6-ol α-Tocopherol 60-12-8 2-Phenylethanol Phenylethyl alcohol 64-17-5 Ethanol Ethyl alcohol 64-18-6 Methanoic acid Formic acid 64-19-7 Ethanoic acid Ace&c acid 65-85-0 Benzoic acid Benzoic acid 66-25-1 Hexanal C-6 Aldehyde 67-63-0 Propan-2-ol 2-Propanol 67-64-1 Propan-2-one 2-Propanone 75-07-0 Acetaldehyde Acetaldehyde 75-18-3 Methylsulfanylmethane Dimethyl sulfide 75-65-0 2-Methyl-propan-2-ol 2-Methyl-2-propanol 76-22-2 1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one Camphor 77-53-2 (1S,2R,5S,7R,8R)-2,6,6,8-Tetramethyltricyclo[5.3.1.0(1,5)]undecan-8-ol Cedrol 77-54-3 (1S,2R,5S,8R)-2,6,6,8-Tetramethyltricyclo[5.3.1.0(1,5)]undecan-8-yl acetate Cedarwood oil acetylated 77-83-8 Ethyl 3-methyl-3-phenyloxirane-2-carboxylate Ethyl methylphenylglycidate 77-90-7 1,2,3-Tributyl 2-(acetyloxy)propane-1,2,3-tricarboxylate Tributyl o-acetylcitrate 77-92-9 3-Carboxy-3-hydroxypentanedioic acid Citric acid; 2-Hydroxypropane-1,2,3-tricarboxylic acid 77-93-0 1,2,3-Triethyl 2-hydroxypropane-1,2,3-tricarboxylate Triethyl citrate 78-35-3 3,7-Dimethylocta-1,6-dien-3-yl 2-methylpropanoate Linalyl isobutyrate 78-36-4 3,7-Dimethylocta-1,6-dien-3-yl butanoate 1-Ethenyl-1,5-dimethyl-4-hexen-1-yl butanoate 78-37-5 3,7-Dimethylocta-1,6-dien-3-yl (E)-3-phenylprop-2-enoate Linalyl cinnamate 78-69-3 3,7-Dimethyloctan-3-ol Tetrahydrolinalool -

Food and Drug Administration, HHS § 172.515
Food and Drug Administration, HHS § 172.515 [42 FR 14491, Mar. 15, 1977, as amended at 43 FR 14644, Apr. 7, 1978; 49 FR 10104, Mar. 19, 1984; 54 FR 24897, June 12, 1989; 69 FR 24511, May 4, 2004; 72 FR 10357, Mar. 8, 2007] § 172.515 Synthetic flavoring sub- Amyl heptanoate. stances and adjuvants. Amyl hexanoate. Amyl octanoate. Synthetic flavoring substances and Anisole; methoxybenzene. adjuvants may be safely used in food in Anisyl acetate. accordance with the following condi- Anisyl alcohol; p-methoxybenzyl alcohol. tions. Anisyl butyrate (a) They are used in the minimum Anisyl formate. Anisyl phenylacetate. quantity required to produce their in- Anisyl propionate. tended effect, and otherwise in accord- Beechwood creosote. ance with all the principles of good Benzaldehyde dimethyl acetal. manufacturing practice. Benzaldehyde glyceryl acetal; 2-phenyl-m-di- (b) They consist of one or more of the oxan-5-ol. following, used alone or in combination Benzaldehyde propylene glycol acetal; 4- with flavoring substances and adju- methyl-2-phenyl-m-dioxolane. vants generally recognized as safe in Benzenethiol; thiophenol. Benzoin; 2-hydroxy-2-phenylacetophenone. food, prior-sanctioned for such use, or Benzophenone; diphenylketone. regulated by an appropriate section in Benzyl acetate. this part. Benzyl acetoacetate. Benzyl alcohol. Acetal; acetaldehyde diethyl acetal. Benzyl benzoate. Acetaldehyde phenethyl propyl acetal. Benzyl butyl ether. ′ Acetanisole; 4 -methoxyacetophenone. Benzyl butyrate. Acetophenone; methyl phenyl ketone. Benzyl cinnamate. Allyl anthranilate. Benzyl 2,3–dimethylcrotonate; benzyl methyl Allyl butyrate. tiglate. Allyl cinnamate. Benzyl disulfide; dibenzyl disulfide. Allyl cyclohexaneacetate. Benzyl ethyl ether. Allyl cyclohexanebutyrate. Benzyl formate. Allyl cyclohexanehexanoate. 3-Benzyl-4-heptanone; benzyl dipropyl ke- Allyl cyclohexaneproprionate. -

This Document Is a Postprint Version of an Article Published in Food Chemistry © Elsevier After Peer Review. to Access The
This document is a postprint version of an article published in Food Chemistry © Elsevier after peer review. To access the final edited and published work see https://doi.org/10.1016/j.foodchem.2019.125555. Document downloaded from: Use of a flor yeast strain for the second fermentation of sparkling wines: effect of endogenous CO2 over-pressure on the volatilome Running Title: Use of a flor yeast strain for the diversification of sparkling wines by Rafael Martínez-Garcíaa, Yenifer Roldán-Romerob, Juan Morenoa*, Anna Puig-Pujolc, Juan Carlos Mauriciob and Teresa García-Martínezb a Department of Agricultural Chemistry, Marie Curie (C3) building, Agrifood Campus of International Excellence CeiA3, University of Córdoba, Ctra. N-IV-A, km 396, 14014 Cordoba, Spain b Department of Microbiology, Severo Ochoa (C6) building, Agrifood Campus of International Excellence CeiA3, University of Cordoba, Ctra. N-IV-A, kmm 396, 14014 Cordoba, Spain. cInstitut de Recerca i Tecnologia Agroalimentaries - Institut Català de la Vinya i el Vi). Plaça Àgora, 2. 08720 Vilafranca del Penedès (Barcelona), España. *Corresponding author E-mail address: [email protected] (J. Moreno). 1 ABSTRACT Saccharomyces cerevisiae flor yeast is used for the first time in sparkling wine-making. Twenty- six oenological variables and fifty-three volatile metabolites are quantified in the middle (P = 3 bar) and at the end (P = 6 bar) of the second fermentation, carried out in open and closed bottles. A heat- map of volatiles and the fingerprints obtained for ten chemical families and ten odorant series visualize the changes for each condition. Terpenes, fatty acids and volatile phenols increased their contents by pressure effect at the end of the study by 25.0, 7.8 and 2.2 %, respectively.