Ribonuclease A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ribonuclease A: Disulfide Bonds, Conformational Stability, and Cytotoxicity
Ribonuclease A: Disulfide Bonds, Conformational Stability, and Cytotoxicity by Tony A. Klink A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Biochemistry) at the UNIVERSITY OF WISCONSIN-MADISON 2()()() r OJ A dissertation entitled Ribonuclease A: Disulfide Bonds, Conformational Stability and Cytotoxicity submitted to the Graduate School of the University of Wisconsin-Madison in partial fulfillment of the requirements for the degree of Doctor of Philosophy by Tony Anthony Klink Date of Final Oral Examination: August 4, 2000 Month & Year Degree to be awarded: December May August 2000 • * * * * * * • * * • * • • • • • * • • • * • • • • • • • • • • • • • * * • * • * * * • * • * • • • • • • * App oval Signature. f Dissertation Readers: Signature, Dean of Graduate School ---..., v.C~vJ,1A. 5. lJ,~k;at 1 Abstract Disulfide bonds between the side chains of cysteine residues are the only common cross links in proteins. Bovine pancreatic ribonuclease A (RNase A) is a 124-residue enzyme that contains four interweaving disulfide bonds (Cys26-Cys84, Cys40-Cys95, Cys58-CysllO, and Cys65-Cys72) and catalyzes the cleavage of RNA. The contribution of each disulfide bond to the confonnational stability and catalytic activity of RNase A was detennined using variants in which each cystine was replaced independently with a pair of alanine residues. Of the four disulfide bonds, the Cys40-Cys95 and Cys65-Cys72 cross-links are the least important to confonnational stability. Removing these disulfide bonds leads to RNase A variants that have Tm values below that of the wild-type enzyme but above physiological temperature. Unlike wild-type RNase A. G88R RNase A is toxic to cancer cells. To investigate the relationship between conformational stability and cytotoxicity, the C40AlC95A and C65A1C72A variants were made in the G88R background. -

Determination of Pk, Values of the Histidine Side
Protein Science (1997), 6:1937-1944. Cambridge University Press. Printed in the USA Copyright 0 1997 The Protein Society Determination of pK, values of the histidine side chains of phosphatidylinositol-specific phospholipase C from Bacillus cereus by NMR spectroscopy and site-directed mutagenesis TUN LIU, MARGRET RYAN, FREDERICK W. DAHLQUIST, AND 0. HAYES GRIFFITH Institute of Molecular Biology and Department of Chemistry, University of Oregon, Eugene, Oregon 97403 (RECEIVEDDecember 4, 1996: ACCEPTEDMay 19, 1997) Abstract Two active site histidine residues have been implicated in the catalysis of phosphatidylinositol-specific phospholipase C (PI-PLC). In this report, we present the first study of the pK,, values of histidines of a PI-PLC. All six histidines of Bacillus cereus PI-PLC were studied by 2D NMR spectroscopy and site-directed mutagenesis. The protein was selec- tively labeled with '3C"-histidine. A series of 'H-I3C HSQC NMR spectra were acquired over a pH range of 4.0-9.0. Five of the six histidines have been individually substituted with alanine to aid the resonance assignments in the NMR spectra. Overall, the remaining histidines in the mutants show little chemical shift changes in the 'H-"C HSQC spectra, indicating that the alanine substitution has no effect on the tertiary structure of the protein. H32A and H82A mutants are inactive enzymes, while H92A and H61A are fully active, and H81A retains about 15% of the wild-type activity. The active site histidines, His32 and His82, display pK,, values of 7.6 and 6.9, respectively. His92 and His227 exhibit pK, values of 5.4 and 6.9. -
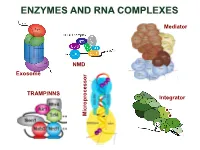
Enzymes and Rna Complexes
ENZYMES AND RNA COMPLEXES Mediator NMD Exosome NMD TRAMP/NNS Integrator Microprocessor RNA PROCESSING and DECAY machinery: RNases Protein Function Characteristics Exonucleases 5’ 3’ Xrn1 cytoplasmic, mRNA degradation processsive Rat1 nuclear, pre-rRNA, sn/snoRNA, pre-mRNA processing and degradation Rrp17/hNol12 nuclear, pre-rRNA processing Exosome 3’ 5’ multisubunit exo/endo complex subunits organized as in bacterial PNPase Rrp44/Dis3 catalytic subunit Exo/PIN domains, processsive Rrp4, Rrp40 pre-rRNA, sn/snoRNA processing, mRNA degradation Rrp41-43, 45-46 participates in NMD, ARE-dependent, non-stop decay Mtr3, Ski4 Mtr4 nuclear helicase cofactor DEAD box Rrp6 (Rrp47) nuclear exonuclease ( Rrp6 BP, cofactor) RNAse D homolog, processsive Ski2,3,7,8 cytoplasmic exosome cofactors. SKI complex helicase, GTPase Other 3’ 5’ Rex1-4 3’-5’ exonucleases, rRNA, snoRNA, tRNA processing RNase D homolog DXO 3’-5’ exonuclease in addition to decapping mtEXO 3’ 5’ mitochondrial degradosome RNA degradation in yeast Suv3/ Dss1 helicase/ 3’-5’ exonuclease DExH box/ RNase II homolog Deadenylation Ccr4/NOT/Pop2 major deadenylase complex (Ccr, Caf, Pop, Not proteins) Ccr4- Mg2+ dependent endonuclease Pan2p/Pan3 additional deadenylases (poliA tail length) RNase D homolog, poly(A) specific nuclease PARN mammalian deadenylase RNase D homolog, poly(A) specific nuclease Endonucleases RNase III -Rnt1 pre-rRNA, sn/snoRNA processing, mRNA degradation dsRNA specific -Dicer, Drosha siRNA/miRNA biogenesis, functions in RNAi PAZ, RNA BD, RNase III domains Ago2 Slicer -

Rnase 2 Sirna (H): Sc-92235
SANTA CRUZ BIOTECHNOLOGY, INC. RNase 2 siRNA (h): sc-92235 BACKGROUND PRODUCT RNase 2 [ribonuclease, RNase A family, 2 (liver, eosinophil-derived neuro- RNase 2 siRNA (h) is a pool of 2 target-specific 19-25 nt siRNAs designed toxin)], also known as non-secretory ribonuclease, EDN (eosinophil-derived to knock down gene expression. Each vial contains 3.3 nmol of lyophilized neurotoxin), RNase UpI-2 or RNS2, is a 161 amino acid protein that belongs siRNA, sufficient for a 10 µM solution once resuspended using protocol to the pancreatic ribonuclease family. Localizing to lysosome and cytoplasmic below. Suitable for 50-100 transfections. Also see RNase 2 shRNA granules, RNase 2 is expressed in leukocytes, liver, spleen, lung and body Plasmid (h): sc-92235-SH and RNase 2 shRNA (h) Lentiviral Particles: fluids. RNase 2 functions as a pyrimidine specific nuclease, and has a slight sc-92235-V as alternate gene silencing products. preference for uracil. RNase 2 is capable of various biological activities, For independent verification of RNase 2 (h) gene silencing results, we including mediation of chemotactic activity and endonucleolytic cleavage of also provide the individual siRNA duplex components. Each is available as nucleoside 3'-phosphates and 3'-phosphooligonucleotides. The gene encoding 3.3 nmol of lyophilized siRNA. These include: sc-92235A and sc-92235B. RNase 2 maps to human chromosome 14q11.2. STORAGE AND RESUSPENSION REFERENCES Store lyophilized siRNA duplex at -20° C with desiccant. Stable for at least 1. Yasuda, T., Sato, W., Mizuta, K. and Kishi, K. 1988. Genetic polymorphism one year from the date of shipment. -

Characterization of the Mammalian RNA Exonuclease 5/NEF-Sp As a Testis-Specific Nuclear 3′′′′′ → 5′′′′′ Exoribonuclease
Downloaded from rnajournal.cshlp.org on October 7, 2021 - Published by Cold Spring Harbor Laboratory Press Characterization of the mammalian RNA exonuclease 5/NEF-sp as a testis-specific nuclear 3′′′′′ → 5′′′′′ exoribonuclease SARA SILVA,1,2 DAVID HOMOLKA,1 and RAMESH S. PILLAI1 1Department of Molecular Biology, University of Geneva, CH-1211 Geneva 4, Switzerland 2European Molecular Biology Laboratory, Grenoble Outstation, 38042, France ABSTRACT Ribonucleases catalyze maturation of functional RNAs or mediate degradation of cellular transcripts, activities that are critical for gene expression control. Here we identify a previously uncharacterized mammalian nuclease family member NEF-sp (RNA exonuclease 5 [REXO5] or LOC81691) as a testis-specific factor. Recombinant human NEF-sp demonstrates a divalent metal ion-dependent 3′′′′′ → 5′′′′′ exoribonuclease activity. This activity is specific to single-stranded RNA substrates and is independent of their length. The presence of a 2′′′′′-O-methyl modification at the 3′′′′′ end of the RNA substrate is inhibitory. Ectopically expressed NEF-sp localizes to the nucleolar/nuclear compartment in mammalian cell cultures and this is dependent on an amino-terminal nuclear localization signal. Finally, mice lacking NEF-sp are viable and display normal fertility, likely indicating overlapping functions with other nucleases. Taken together, our study provides the first biochemical and genetic exploration of the role of the NEF-sp exoribonuclease in the mammalian genome. Keywords: NEF-sp; LOC81691; Q96IC2; REXON; RNA exonuclease 5; REXO5; 2610020H08Rik INTRODUCTION clease-mediated processing to create their final 3′ ends: poly(A) tails of most mRNAs or the hairpin structure of Spermatogenesis is the process by which sperm cells are replication-dependent histone mRNAs (Colgan and Manley produced in the male germline. -

Protein Engineering to Exploit and Explore Bovine Secretory Ribonucleases
PROTEIN ENGINEERING TO EXPLOIT A N D EXPLORE BOVINE SECRETORY RIBONUCLEASES by JIN-SOO KIM A thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Biochemistry) at the UNIVERSITY OF WISCONSIN-MADISON 1994 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. ACKNOWLEDGEMENTS I would like to thank Dr. Ronald T. Raines for his advice and support. His scientific insight has been very helpful throughout this work. I would also like to thank the entire Raines group for their friendship and companionship. I am grateful to Dr. J. Soucek and Dr. J. Matousek for their collaboration with us, which has been a valuable part of the BS-RNase research. I thank Dr. M. Karpeisky for suggesting the protein fusion project, and Dr. G. D'Alessio and Dr. L. Mazzarella for providing the coordinates of BS-RNase. I have been generously supported by Steenbock predoctoral fellowship from the Department of Biochemistry. Finally, I thank my parents, who have encouraged (or at least not discouraged) me to pursue a career in science since I was a kid. Reproduced with permission of the copyright owner. Further reproduction prohibited without permission ABSTRACT PROTEIN ENGINEERING TO EXPLOIT AND EXPLORE BOVINE SECRETORY RIBONUCLEASES Jin-Soo Kim Under the supervision of Dr. Ronald T. Raines at the University of Wisconsin-Madison Ribonuclease S-peptide (residues 1-20) and S-protein (residues 21- 124) are the enzymatically inactive products of the limited digestion of bovine pancreatic ribonuclease A (RNase A) by subtilisin. S-Peptide binds S-protein with high affinity to form RNase S, which has full enzymatic activity. -

United States Patent (19) 11) 4,039,382 Thang Et Al
United States Patent (19) 11) 4,039,382 Thang et al. 45 Aug. 2, 1977 54 MMOBILIZED RIBONUCLEASE AND -Enzyme Systems, Journal of Food Science, vol. 39, ALKALINE PHOSPHATASE 1974, (pp. 647-652). 75 Inventors: Minh-Nguy Thang, Bagneux; Annie Zaborsky, O., Immobilized Enzymes, CRC Press, Guissani born Trachtenberg, Fresnes, Cleveland, Ohio, 1973, (pp. 124-126). both of France Primary Examiner-David M. Naff 73 Assignee: Choay S. A., Paris, France Attorney, Agent, or Firm-Browdy and Neimark 21 Appl. No.: 678,459 22 Filed: Apr. 19, 1976 57 ABSTRACT An insoluble, solid matrix carrying simultaneously sev 30 Foreign Application Priority Data eral different enzymatic functions, is constituted by the Apr. 23, 1975 France ................................ 75.12667 conjoint association by irreversible binding on a previ ously activated matrix support, of a nuclease selected 51) int. Cl? ........................... C07G 7/02; C12B 1/00 from the group of ribonucleases A, T, T, U, and an 52 U.S. Cl. ................................... 195/28 N; 195/63; alkaline phosphatease. Free activated groups of the 195/68; 195/DIG. 11; 195/116 matrix after binding of the enzymes, are neutralized by 58 Field of Search ................... 195/63, 68, DIG. 11, a free amino organic base. The support is selected from 195/116, 28 N among non-denaturing supports effecting the irrevers 56) References Cited ible physical adsorption of the enzymes, such as sup ports of glass or quartz beads, highly cross-linked gels PUBLICATIONS of the agarose or cellulose type. Polymers AUott, A. Lee, J. C., Preparation and Properties of Water Insolu Cott, AGot and/or oligonucleotides U, C, A or G, of ble Derivatives of Ribonuclease Ti. -

The Characterization of Oligonucleotides and Nucleic Acids Using Ribonuclease H and Mass Spectrometry
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1999 The hC aracterization of Oligonucleotides and Nucleic Acids Using Ribonuclease H and Mass Spectrometry. Lenore Marie Polo Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Polo, Lenore Marie, "The hC aracterization of Oligonucleotides and Nucleic Acids Using Ribonuclease H and Mass Spectrometry." (1999). LSU Historical Dissertations and Theses. 6923. https://digitalcommons.lsu.edu/gradschool_disstheses/6923 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter free, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. -

Linked 5' Nucleotide Sequence at the 5' End of Rabbit Globin Messenger Ribonucleic Acid by JOHN A
Biochem. J. (1976) 155, 637-644 637 Printed in Great Britain The Nature of the 5'-Linked 5' Nucleotide Sequence at the 5' End of Rabbit Globin Messenger Ribonucleic Acid By JOHN A. HUNT and GARY N. OAKES Department of Geneties, John A. Burns School ofMedicine, University ofHawaii, Honolulu, HI 96822, U.S.A. (Received 15 January 1976) Poly(A)-containing messenger RNA isolated from rabbit reticulocytes as estimated by periodate oxidation and condensation with [3H]isoniazid has two oxidizable end groups per molecule of mol.wt. 220000. When the mRNA is subjected to stepwise degradation by fl-elimination, only one oxidizable end-group is found. This indicates that one of the 2',3'hydroxyl end-groups is linked through the normal 3'-5' phosphodiester bond, butthat the other is linked in such a way that after stepwise degradation no new 2',3' hydroxyl group is revealed. This structure could be a 5'-linked 5'-phospho di- or tri-ester. On digestion with ribonuclease the isoniazid-labelled RNA produced oligonucleotide hydrazones consistent with a poly(A) sequence at the 3' end plus fragnents that are not found after stepwise degradation. These fragments have a charge of -6 and -8 from pancreatic ribonuclease or -7 from ribonuclease T1 digestion. These charges are changed to -3.4 and -4.1 after pancreatic ribonuclease, ribonuclease T2 and alkaline phosphatase digestion. methyl-3H-labelled-poly(A)-containing RNA isolated from late erythroid cells contain a methyl-labelled fragment resistant to endonuclease and phosphodiesterase II digestion. After digestion with phosphodiesterase I this fragment produces methyl-3H- labelled nucleotides with the electrophoretic mobility of pm7G and pAm. -

Insights Into the Structure and Architecture of the CCR4–NOT Complex
REVIEW ARTICLE published: 16 May 2014 doi: 10.3389/fgene.2014.00137 Insights into the structure and architecture of the CCR4–NOT complex Kun Xu 1,2 ,Yuwei Bai 1, Aili Zhang 1,2, Qionglin Zhang 2 and Mark G. Bartlam1,2* 1 State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, China 2 College of Life Sciences, Nankai University, Tianjin, China Edited by: The CCR4–NOT complex is a highly conserved, multifunctional machinery with a general Martine Anne Collart, University of role in controlling mRNA metabolism. It has been implicated in a number of different Geneva, Switzerland aspects of mRNA and protein expression, including mRNA degradation, transcription Reviewed by: initiation and elongation, ubiquitination, and protein modification. The core CCR4–NOT Walter Lukiw, Louisiana State University, USA complex is evolutionarily conserved and consists of at least three NOT proteins and two Sebastiaan Winkler, University of catalytic subunits.The L-shapedcomplex is characterized by two functional modules bound Nottingham, UK to the CNOT1/Not1 scaffold protein: the deadenylase or nuclease module containing *Correspondence: two enzymes required for deadenylation, and the NOT module. In this review, we will Mark G. Bartlam, College of Life summarize the currently available information regarding the three-dimensional structure Sciences, Nankai University, 94 Weijin Road, Tianjin 300071, China and assembly of the CCR4–NOT complex, in order to provide insight into its roles in mRNA e-mail: [email protected] degradation and other biological processes. Keywords: CCR4–NOT complex, mRNA decay, poly(A), deadenylation, NOT module, protein structure INTRODUCTION 0.9–1.2 MDa in size (Liu et al., 1998). -

Signature Redacted Certified By: Ronald T
Endogenous and Chemical Modifications of Model Proteins by Valerie T. Ressler B.A. Chemistry Macalester College, 2011 M.S. Chemistry College of William & Mary, 2013 Submitted to the Department of Chemistry in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY IN CHEMISTRY at the Massachusetts Institute of Technology February 2019 @ 2019 Massachusetts Institute of Technology. All rights reserved. Signature Signature of Author: redacted____ Department of Chemistry January 14, 2019 -Signature redacted Certified by: Ronald T. Raines Firmeni Pro essor of Chemistry hesis Supervisor Signature redacted Accepted by: Robert W. Field OFTECHNOWGO Haslam and Dewey Professor of Chemistry Chairman, Departmental Committee on Graduate Students MAR 21 2019 LIBRARIES ARCHIVES 1 This doctoral thesis has been examined by a committee of professors from the Department of Chemistry as follows: Signature redacted Matthew D. Shoulders Whitehead CD Associate Professor Thesis Committee Chair Signature redacted Ronald T. Raines Firmenich Professor of Chemistry I A Thesis Supervisor Signature redacted Laura L. Kiessling Ne~ovartis Professor of Chemistry Thesis Committee Member 2 Endogenous and Chemical Modifications of Model Proteins by Valerie T. Ressler Submitted to the Department of Chemistry on January 15, 2019 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Chemistry Abstract Protein modifications are ubiquitous in nature, introducing biological complexity and functional diversity. Of the known post-translational modifications, glycosylation is one of the most common and most complex, yet some of the biological implications of this modification remain poorly understood. The development of chemical tools to mimic these modifications is helping to elucidate their biological roles and improve the range of biopharmaceuticals. -

Editorial What Is the Role of the Eosinophil?
161 Thorax 1990;45:161-3 THORAX Thorax: first published as 10.1136/thx.45.3.161 on 1 March 1990. Downloaded from Editorial What is the role of the eosinophil? The eosinophil granulocyte was first described in blood in Eosinophil cationic protein Eosinophil cationic protein 1879 by the German scientist Paul Ehrlich. During the is a heterogeneous but single chained protein with a following decades study of the eosinophil attracted many molecular weight that ranges from 18 to 21 kD. The investigators, who showed among other things that high primary amino acid sequence ofeosinophil cationic protein eosinophil counts in blood were associated with diseases shows substantial homology with eosinophil protein X, such as asthma and with parasite infestations.' 2 It was even angiogenin, and pancreatic ribonuclease. This last concluded that the massive tissue eosinophilia found in homology suggested that eosinophil cationic protein might patients dying from status asthmaticus pointed to a role for have ribonuclease activity, and this has been confirmed.2 22 the eosinophil in asthma and that understanding the The homology with eosinophil protein X indicated a close mechanisms concerned in the creation of tissue eosino- relation between the two proteins and might explain why philia "... would undoubtedly greatly elucidate the one of the monoclonal antibodies, EG2, produced against pathogenesis of asthma." Despite such statements interest eosinophil cationic protein also reacts with eosinophil -in the eosinophil faded and few publications contributing protein X.2" Besides being a weak ribonuclease eosinophil to our understanding of this cell emerged during the cationic protein has several other interesting biological following 50 years.