Gene Dosage: Evidence for Assignment of Erythrocyte Acid Phosphatase Locus to Chromosome 2 (Trisomy/Translocation/Polymorphism/Malformations) R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PFK-2/Fbpase-2: Maker and Breaker of the Essential Biofactor Fructose-2, 6-Bisphosphate
30 Review TRENDS in Biochemical Sciences Vol.26 No.1 January 2001 PFK-2/FBPase-2: maker and breaker of the essential biofactor fructose-2, 6-bisphosphate DavidA. Okar, Ànna Manzano,Aurèa Navarro-Sabatè, Lluìs Riera, Ramon Bartrons and Alex J. Lange Fructose-2,6-bisphosphate is responsible for mediating glucagon-stimulated extraction in base led to the discovery of F-2,6-P2 and gluconeogenesis in the liver.This discovery has led to the realization that this the realization that it was either formed or destroyed compound plays a significant role in directing carbohydrate fluxes in all during metabolic transitions where its concentration eukaryotes. Biophysical studies of the enzyme that both synthesizes and determined changes in glycolytic and gluconeogenic degrades this biofactor have yielded insight into its molecular enzymology. carbon flux in the liver. Interest in this compound grew Moreover, the metabolic role of fructose-2,6-bisphosphate has great potential rapidly because it was found to be a most potent in the treatment of diabetes. positive allosteric effector of 6-phosphofructo-1- kinase (PFK-1; EC 2.7.1.11) and an inhibitor of A trail that began with the discovery of a highly potent fructose-1,6-bisphosphatase (FBPase-1; EC 3.1.3.11). regulator of mammalian hepatic carbohydrate Because of its antagonistic actions on these enzymes, metabolism, fructose-2,6-bisphosphate (F-2,6-P2), led F-2,6-P2 plays a crucial role in the control of the to the discovery of the bifunctional enzyme opposing hepatic glycolytic and gluconeogenic 6-phosphofructo-2-kinase/fructose- pathways1–4 (Fig. -

Structural Study of the Acid Sphingomyelinase Protein Family
Structural Study of the Acid Sphingomyelinase Protein Family Alexei Gorelik Department of Biochemistry McGill University, Montreal August 2017 A thesis submitted to McGill University in partial fulfillment of the requirements of the degree of Doctor of Philosophy © Alexei Gorelik, 2017 Abstract The acid sphingomyelinase (ASMase) converts the lipid sphingomyelin (SM) to ceramide. This protein participates in lysosomal lipid metabolism and plays an additional role in signal transduction at the cell surface by cleaving the abundant SM to ceramide, thus modulating membrane properties. These functions are enabled by the enzyme’s lipid- and membrane- interacting saposin domain. ASMase is part of a small family along with the poorly characterized ASMase-like phosphodiesterases 3A and 3B (SMPDL3A,B). SMPDL3A does not hydrolyze SM but degrades extracellular nucleotides, and is potentially involved in purinergic signaling. SMPDL3B is a regulator of the innate immune response and podocyte function, and displays a partially defined lipid- and membrane-modifying activity. I carried out structural studies to gain insight into substrate recognition and molecular functions of the ASMase family of proteins. Crystal structures of SMPDL3A uncovered the helical fold of a novel C-terminal subdomain, a slightly distinct catalytic mechanism, and a nucleotide-binding mode without specific contacts to their nucleoside moiety. The ASMase investigation revealed a conformational flexibility of its saposin domain: this module can switch from a detached, closed conformation to an open form which establishes a hydrophobic interface to the catalytic domain. This open configuration represents the active form of the enzyme, likely allowing lipid access to the active site. The SMPDL3B structure showed a narrow, boot-shaped substrate binding site that accommodates the head group of SM. -

The Role of Triacylglycerol in Plant Stress Response
plants Review The Role of Triacylglycerol in Plant Stress Response Junhao Lu 1, Yang Xu 1, Juli Wang 1, Stacy D. Singer 2,* and Guanqun Chen 1,* 1 Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, T6G 2P5 Alberta, Canada; [email protected] (J.L.); [email protected] (Y.X.); [email protected] (J.W.) 2 Agriculture and Agri-Food Canada, Lethbridge Research and Development Centre, Lethbridge, T1J 4B1 Alberta, Canada * Correspondence: [email protected] (S.D.S.); [email protected] (G.C.); Tel.: +1-403-317-3386 (S.D.S.); +1-780-492-3148 (G.C.) Received: 4 March 2020; Accepted: 2 April 2020; Published: 8 April 2020 Abstract: Vegetable oil is mainly composed of triacylglycerol (TAG), a storage lipid that serves as a major commodity for food and industrial purposes, as well as an alternative biofuel source. While TAG is typically not produced at significant levels in vegetative tissues, emerging evidence suggests that its accumulation in such tissues may provide one mechanism by which plants cope with abiotic stress. Different types of abiotic stress induce lipid remodeling through the action of specific lipases, which results in various alterations in membrane lipid composition. This response induces the formation of toxic lipid intermediates that cause membrane damage or cell death. However, increased levels of TAG under stress conditions are believed to function, at least in part, as a means of sequestering these toxic lipid intermediates. Moreover, the lipid droplets (LDs) in which TAG is enclosed also function as a subcellular factory to provide binding sites and substrates for the biosynthesis of bioactive compounds that protect against insects and fungi. -

The Crystal Structure of the Bifunctional Enzyme 6-Phosphofructo-2-Kinase
Research Article 1017 The crystal structure of the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase reveals distinct domain homologies Charles A Hasemann†*, Eva S Istvan1, Kosaku Uyeda1,2 and Johann Deisenhofer1,3 Background: Glucose homeostasis is maintained by the processes of Addresses: 1Department of Biochemistry, glycolysis and gluconeogenesis. The importance of these pathways is University of Texas Southwestern Medical Center, demonstrated by the severe and life threatening effects observed in various 5323 Harry Hines Blvd., Dallas, TX 75235 USA, 2Research Service, Department of Veterans Affairs forms of diabetes. The bifunctional enzyme 6-phosphofructo-2-kinase/fructose- Medical Center, 4500 S. Lancaster Rd., Dallas, 2,6-bisphosphatase catalyzes both the synthesis and degradation of fructose- TX, 75216 USA and 3Howard Hughes Medical 2,6-bisphosphate, a potent regulator of glycolysis. Thus this bifunctional enzyme Institute, University of Texas, Southwestern plays an indirect yet key role in the regulation of glucose metabolism. Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75235, USA. Results: We have determined the 2.0 Å crystal structure of the rat testis †Present address: Department of Internal isozyme of this bifunctional enzyme. The enzyme is a homodimer of 55 kDa Medicine, Division of Rheumatology, and the subunits arranged in a head-to-head fashion, with each monomer consisting of Harold C Simmons Center for Arthritis Research, University of Texas Southwestern Medical Center, independent kinase and phosphatase domains. The location of ATPgS and 5323 Harry Hines Blvd., Dallas, TX 75235-8884, inorganic phosphate in the kinase and phosphatase domains, respectively, allow USA. us to locate and describe the active sites of both domains. -

Genomics of Inherited Bone Marrow Failure and Myelodysplasia Michael
Genomics of inherited bone marrow failure and myelodysplasia Michael Yu Zhang A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2015 Reading Committee: Mary-Claire King, Chair Akiko Shimamura Marshall Horwitz Program Authorized to Offer Degree: Molecular and Cellular Biology 1 ©Copyright 2015 Michael Yu Zhang 2 University of Washington ABSTRACT Genomics of inherited bone marrow failure and myelodysplasia Michael Yu Zhang Chair of the Supervisory Committee: Professor Mary-Claire King Department of Medicine (Medical Genetics) and Genome Sciences Bone marrow failure and myelodysplastic syndromes (BMF/MDS) are disorders of impaired blood cell production with increased leukemia risk. BMF/MDS may be acquired or inherited, a distinction critical for treatment selection. Currently, diagnosis of these inherited syndromes is based on clinical history, family history, and laboratory studies, which directs the ordering of genetic tests on a gene-by-gene basis. However, despite extensive clinical workup and serial genetic testing, many cases remain unexplained. We sought to define the genetic etiology and pathophysiology of unclassified bone marrow failure and myelodysplastic syndromes. First, to determine the extent to which patients remained undiagnosed due to atypical or cryptic presentations of known inherited BMF/MDS, we developed a massively-parallel, next- generation DNA sequencing assay to simultaneously screen for mutations in 85 BMF/MDS genes. Querying 71 pediatric and adult patients with unclassified BMF/MDS using this assay revealed 8 (11%) patients with constitutional, pathogenic mutations in GATA2 , RUNX1 , DKC1 , or LIG4 . All eight patients lacked classic features or laboratory findings for their syndromes. -
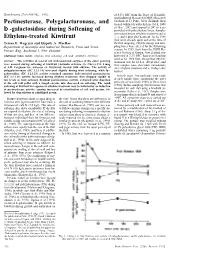
Pectinesterase, Polygalacturonase
HORTSCIENCE 27(8):900-902. 1992. of 8.2% SSC from the Dept. of Scientific and Industrial Research (DSIR) Research Orchard at Te Puke, New Zealand, then Pectinesterase, Polygalacturonase, and treated within 6 h with ethylene (16 h, 1000 µl·liter-1, 20C) and ripened at 20C in trays b -galactosidase during Softening of provided with polyethylene liners. Samples were taken before ethylene treatment and at Ethylene-treated Kiwifruit 1, 2, and 5 days after treatment. As the 1988 fruit were already quite soft at the time of Teresa F. Wegrzyn and Elspeth A. MacRae the first sampling, ethylene dosage and sam- Department of Scientific and Industrial Research, Fruit and Trees, pling times were altered for the following Private Bag, Auckland I, New Zealand season. In 1989, fruit from the DSIR Re- search Orchard at Kumeu, New Zealand, was Additional index words. ethylene, fruit softening, cell wall, Actinidia deliciosa harvested at 7.2% SSC, then treated and rip ened as for 1988 fruit, except that ethylene Abstract. The activities of several cell wall-associated enzymes of the outer pericarp treatment was for 18 h at 100 µl·liter-1, and were assayed during softening of kiwifruit [Actinidia deliciosa (A. Chev.) C.F. Liang fruit samples were also taken immediately et A.R. Ferguson var. deliciosa cv. Hayward] treated with ethylene. The activity of after ethylene treatment and at 10 days after polygalacturonase (EC 3.2.1.15) increased slightly during fruit softening, while b- harvest. galactosidase (EC 3.2.1.23) activity remained constant. Salt-extracted pectinesterase (EC 3.1.1.11) activity increased during ethylene treatment, then dropped rapidly to In both years, five replicates were made low levels as fruit softened. -

A Review of Phosphatidate Phosphatase Assays
REVIEW A review of phosphatidate phosphatase assays Prabuddha Dey1, Gil-Soo Han1 , and George M. Carman1,* 1Department of Food Science and the Rutgers Center for Lipid Research, New Jersey Institute for Food, Nutrition, and Health, Rutgers University, New Brunswick, NJ, USA Abstract Phosphatidate phosphatase (PAP) catalyzes the ganisms studied thus far, PAP activity is dependent on the penultimate step in the synthesis of triacylglycerol and regu- DXDX(T/V) catalytic motif in the haloacid dehalogenase- lates the synthesis of membrane phospholipids. There is like domain (10, 13, 14). The enzyme is extensively modi- much interest in this enzyme because it controls the cellular fied posttranslationally by phosphorylation, which acutely levels of its substrate, phosphatidate (PA), and product, regulates its catalytic activity, subcellular localization, and DAG; defects in the metabolism of these lipid intermediates are the basis for lipid-based diseases such as obesity, lipodys- protein stability (15–17). Downloaded from trophy, and inflammation. The measurement of PAP activity is PAP is a key lipid metabolic enzyme that is required for required for studies aimed at understanding its mechanisms the synthesis of the neutral lipid triacylglycerol (TAG) and of action, how it is regulated, and for screening its activators major membrane phospholipids (16–18) (Fig. 1). The en- and/or inhibitors. Enzyme activity is determined through zyme product, DAG, is a direct precursor of TAG (16, 19–22), the use of radioactive and nonradioactive assays that measure whereas its substrate, PA, is a direct precursor of the lipo- the product, DAG, or Pi. However, sensitivity and ease of use nucleotide, CDP-DAG, a key intermediate in phospholipid www.jlr.org are variable across these methods. -

Defensive Forwards: Stress-Responsive Proteins in Cell Walls of Crop Plants
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.15.950535; this version posted February 16, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 Short communication 2 3 Defensive forwards: stress-responsive proteins in cell walls of crop plants 4 5 Liangjie Niu, Wei Wang* 6 7 National Key Laboratory of Wheat & Maize Crop Science, College of Life Sciences, Henan 8 Agricultural University, Zhengzhou 450002, China 9 10 *Corresponding author. Wei Wang, College of Life Sciences, Henan Agricultural University, 11 Zhengzhou 450002, China. E-mail:[email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.02.15.950535; this version posted February 16, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 12 ABSTRACT 13 As the vital component of plant cell wall, proteins play important roles in stress response 14 through modifying wall structure and involving in wall integrity signaling. However, the 15 potential of cell wall proteins (CWPs) in improvement of crop stress tolerance has probably 16 been underestimated. Recently, we have critically reviewed the predictors, databases and 17 cross-referencing of subcellular locations of possible CWPs in plants (Briefings in 18 Bioinformatics 2018;19:1130-1140). -

The Role of Chloroplast Membrane Lipid Metabolism in Plant Environmental Responses
cells Review The Role of Chloroplast Membrane Lipid Metabolism in Plant Environmental Responses Ron Cook 1,2,†, Josselin Lupette 1,†,‡ and Christoph Benning 1,2,3,* 1 MSU-DOE Plant Research Laboratory, Michigan State University, East Lansing, MI 48824-1319, USA; [email protected] (R.C.); [email protected] (J.L.) 2 Department of Biochemistry and Molecular Biology, Michigan State University, East Lansing, MI 48824-1319, USA 3 Department of Plant Biology, Michigan State University, East Lansing, MI 48824-1319, USA * Correspondence: [email protected] † These authors contributed equally to this work. ‡ Present address: Laboratoire de Biogenèse Membranaire, Université de Bordeaux, CNRS, UMR 5200, F-33140 Villenave d’Ornon, France. Abstract: Plants are nonmotile life forms that are constantly exposed to changing environmental conditions during the course of their life cycle. Fluctuations in environmental conditions can be drastic during both day–night and seasonal cycles, as well as in the long term as the climate changes. Plants are naturally adapted to face these environmental challenges, and it has become increasingly apparent that membranes and their lipid composition are an important component of this adaptive response. Plants can remodel their membranes to change the abundance of different lipid classes, and they can release fatty acids that give rise to signaling compounds in response to environmental cues. Chloroplasts harbor the photosynthetic apparatus of plants embedded into one of the most extensive membrane systems found in nature. In part one of this review, we focus on changes in chloroplast membrane lipid class composition in response to environmental changes, and in part two, we will detail chloroplast lipid-derived signals. -

Induced Glycosaminoglycan and Sphingolipid Accumulation
Proc. Natl. Acad. Sci. USA Vol. 77, No. 6, pp. 3700-3704, June 1980 Medical Sciences Experimental animal model for mucopolysaccharidosis: Suramin- induced glycosaminoglycan and sphingolipid accumulation in the rat (neuronal inclusions/lysosomal enzymes/noncompetitive inhibition/ganglioside storage) GEORGE CONSTANTOPOULOS*, SANDRA REESt, B. G. CRAGGt, JOHN A. BARRANGER*, AND ROSCOE 0. BRADY* *Developmental and Metabolic Neurology Branch, National Institute of Neurological and Communicative Disorders and Stroke, National Institutes of Health, Bethesda, Maryland 20205; and tDepartment of Physiology, Monash University, Clayton, Victoria, 3168, Australia Contributed by Roscoe 0. Brady, March 20, 1980 ABSTRACG Intracerebral injection of the trypanocidal drug (Streptomyces griseus protease, B grade) from Calbiochem. suramin in rats caused the formation of membranous neuronal GAG standards were supplied by Martin B. Mathews, Uni- and neuroglial inclusions. Here we show that intravenous ad- were ministration of suramin, 500 mg/kg, to 2-month-old rats causes versity of Chicago. p-Nitrophenyl glycosides purchased a 5- to 8-fold increase of glycosaminoglycan concentration in from Sigma and Research Products International (Elk Grove the liver within 10 days and a 6-fold increase in urinary gly- Village, IL). Phenyl-a-L-iduronide was a gift from B. Weiss- cosaminoglycan excretion. The excess glycosaminoglycans mann of the University of Illinois College of Medicine, and consist of heparan sulfate and dermatan sulfate. Intracerebral 0-(a-L-idopyranosyluronic acid 2-sulfate)-2,5-anhydro-D- injection of 250 Ag of suramin results in a small increase of [3H]mannitol 6-sulfate was prepared from heparin (7). [N- glycosaminoglycan and larger increase of ganglioside Gm2, GM3, and GD3 concentrations in the treated region of the brain. -

United States Patent 19) 11 Patent Number: 5,436,143 Hyman - 45 Date of Patent: Jul
US005436143A United States Patent 19) 11 Patent Number: 5,436,143 Hyman - 45 Date of Patent: Jul. 25, 1995 54 METHOD FORENZYMATICSYNTHESIS OF cleotides', in Oligonucleotide Synthesis: A Practical Ap OLGONUCLEOTIOES proach, M. J. Gait ed., pp. 185-197 (1984). Mudrakovskaya et al., “RNA Ligase of Bacteriophage 76 Inventor: Edward D. Hyman, 2100 Sawmill T4. VII: A solid pahse enymatic synthesis of Rd., Apt. 4-103, River Ridge, La. oligoribonucelotides', Biorg. Khim., 17: 819-822 701.23 (1991). (21) Appl. No.: 995,791 Stuart et al., “Synthesis and Properties of Oligodeox ynucleotides with an AP site at a preselected location', 22 Filed: Dec. 23, 1992 Nulceic Acids Res. 15: 7451-7462 (1987). 51) Int. Cl. .............................................. C12P 19/34 Norton et al., "A ribonuclease specific for 2'-O-Me 52 U.S. C. .................................. 435/91.2; 435/91.1; thyltaed Ribonulceic Acid', J. Biol. Chem. 242: 435/91.21; 435/91.3; 435/91.31; 435/91.5; 2029-2034 (1967). 435/91.51; 435/91.52; 536/24.33; 536/25.3; Eckstein et al., “Phosphorothioates in molecular biol 536/25.31; 935/16: 935/88 ogy”, TIBS 14:97-100 (1989). 58) Field of Search ................... 435/91, 6, 91.1, 91.2, Bryant et al., “Phosphorothioate Substrates for T4 435/91.21, 91.3, 91.31, 91.5, 91.51, 91.52; RNA Ligase', Biochemistry 21:5877-5885 (1982). 536/25.3, 25.6, 24.33, 25.31; 935/16, 88 McLaughlin et al., “Donor Activation in the TS RNA Ligase Reaction', Biochemistry 24: 267-273 (1985). (56) References Cited Ohtsuka et al., “A new method for 3'-labelling of U.S. -

Human Acid Sphingomyelinase Structures Provide Insight to Molecular Basis of Niemann-Pick Disease Yan-Feng Zhou Analytical Discovery Therapeutics
University of Massachusetts Amherst ScholarWorks@UMass Amherst Biochemistry & Molecular Biology Department Biochemistry and Molecular Biology Faculty Publication Series 2016 Human Acid Sphingomyelinase Structures Provide Insight to Molecular Basis of Niemann-Pick Disease Yan-Feng Zhou Analytical Discovery Therapeutics Matthew .C Metcalf University of Massachusetts Amherst Scott .C Garman University of Massachusetts Amherst Tim Edmunds Sanofi Huawei Qiu Sanofi See next page for additional authors Follow this and additional works at: https://scholarworks.umass.edu/biochem_faculty_pubs Part of the Biochemistry Commons Recommended Citation Zhou, Yan-Feng; Metcalf, Matthew C.; Garman, Scott .;C Edmunds, Tim; Qiu, Huawei; and Wei, Ronnie R., "Human Acid Sphingomyelinase Structures Provide Insight to Molecular Basis of Niemann-Pick Disease" (2016). Nature Communications. 8. 10.1038/ncomms13082 This Article is brought to you for free and open access by the Biochemistry and Molecular Biology at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Biochemistry & Molecular Biology Department Faculty Publication Series by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. Authors Yan-Feng Zhou, Matthew C. Metcalf, Scott .C Garman, Tim Edmunds, Huawei Qiu, and Ronnie R. Wei This article is available at ScholarWorks@UMass Amherst: https://scholarworks.umass.edu/biochem_faculty_pubs/8 ARTICLE Received 23 Feb 2016 | Accepted 1 Sep 2016 | Published 11 Oct 2016 DOI: 10.1038/ncomms13082 OPEN Human acid sphingomyelinase structures provide insight to molecular basis of Niemann–Pick disease Yan-Feng Zhou1,w, Matthew C. Metcalf2, Scott C. Garman2, Tim Edmunds1, Huawei Qiu1 & Ronnie R. Wei1 Acid sphingomyelinase (ASM) hydrolyzes sphingomyelin to ceramide and phosphocholine, essential components of myelin in neurons.