Physiological Resistance Alters Behavioral Response of Tetranychus Urticae to Acaricides Adekunle W
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES
US Environmental Protection Agency Office of Pesticide Programs INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES Note: Pesticide tolerance information is updated in the Code of Federal Regulations on a weekly basis. EPA plans to update these indexes biannually. These indexes are current as of the date indicated in the pdf file. For the latest information on pesticide tolerances, please check the electronic Code of Federal Regulations (eCFR) at http://www.access.gpo.gov/nara/cfr/waisidx_07/40cfrv23_07.html 1 40 CFR Type Family Common name CAS Number PC code 180.163 Acaricide bridged diphenyl Dicofol (1,1-Bis(chlorophenyl)-2,2,2-trichloroethanol) 115-32-2 10501 180.198 Acaricide phosphonate Trichlorfon 52-68-6 57901 180.259 Acaricide sulfite ester Propargite 2312-35-8 97601 180.446 Acaricide tetrazine Clofentezine 74115-24-5 125501 180.448 Acaricide thiazolidine Hexythiazox 78587-05-0 128849 180.517 Acaricide phenylpyrazole Fipronil 120068-37-3 129121 180.566 Acaricide pyrazole Fenpyroximate 134098-61-6 129131 180.572 Acaricide carbazate Bifenazate 149877-41-8 586 180.593 Acaricide unclassified Etoxazole 153233-91-1 107091 180.599 Acaricide unclassified Acequinocyl 57960-19-7 6329 180.341 Acaricide, fungicide dinitrophenol Dinocap (2, 4-Dinitro-6-octylphenyl crotonate and 2,6-dinitro-4- 39300-45-3 36001 octylphenyl crotonate} 180.111 Acaricide, insecticide organophosphorus Malathion 121-75-5 57701 180.182 Acaricide, insecticide cyclodiene Endosulfan 115-29-7 79401 -

Best Management Practices for Brown Dog Ticks
Best Management Practices For Brown Dog Ticks immediately seek shelter in cracks or crevices such as baseboards or furniture, but also commonly move en masse up walls and congregate in the corners of ceilings. These larvae eventually find the dog and receive their first blood meal. The larvae go largely unnoticed because they are about the size of a pencil tip. The larvae then drop off into the surrounding area, molt to the nymphal stage, and again seek the dog for a second blood meal. At this point owners occasionally notice the ticks, but they usually go unnoticed. After they molt to the adult stage, the ticks find the dog for a third and final blood meal. Ticks are noticed at this stage for two reasons: 1) Adult females engorge to the size of a raisin and are often located on or near the dog’s head, or 2) adults are seen Heavy infestation of BDT on the ear of a dog. crawling on floors actively looking for the dog. This “predatory” behavior is somewhat unique to ticks. The brown dog tick (BDT) can be a serious Typically, residents do not notice these ticks until they have pest in homes with pets. These Best Management completed a full generation. Often, overlapping generations of Practices are designed to support cooperation ticks occur in homes, so tick numbers can quickly multiply into the thousands. between homeowners and pest management A factor complicating BDT management is the ability of this professionals in order to prevent and control BDTs. tick to survive without a host for several months during each of At left: The black-legged its three life stages, thereby negating the “wait-it-out” strategy of tick (Ixodes scapularis). -

Sound Management of Pesticides and Diagnosis and Treatment Of
* Revision of the“IPCS - Multilevel Course on the Safe Use of Pesticides and on the Diagnosis and Treatment of Presticide Poisoning, 1994” © World Health Organization 2006 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. CONTENTS Preface Acknowledgement Part I. Overview 1. Introduction 1.1 Background 1.2 Objectives 2. Overview of the resource tool 2.1 Moduledescription 2.2 Training levels 2.3 Visual aids 2.4 Informationsources 3. Using the resource tool 3.1 Introduction 3.2 Training trainers 3.2.1 Organizational aspects 3.2.2 Coordinator’s preparation 3.2.3 Selection of participants 3.2.4 Before training trainers 3.2.5 Specimen module 3.3 Trainers 3.3.1 Trainer preparation 3.3.2 Selection of participants 3.3.3 Organizational aspects 3.3.4 Before a course 4. -

Acaricide Mode of Action Classification: a Key to Effective Acaricide Resistance Management Insecticide Resistance Action Committee
Acaricide Mode of Action Classification: A key to effective acaricide resistance management Insecticide Resistance Action Committee www.irac-online.org Introduction Effective IRM strategies: Sequences or alternations of MoA IRAC promotes the use of a Mode of Action (MoA) classification of All effective pesticide resistance management strategies seek to minimise the selection of resistance to any one type of MoA w MoA x MoA y MoA z MoA w MoA x insecticides and acaricides as the basis for effective and sustainable pesticide. In practice, alternations, sequences or rotations of compounds from different MoA groups provide sustainable and resistance management. Acaricides are allocated to specific groups based effective resistance management for acarine pests. This ensures that selection from compounds in the same MoA group is on their target site. Reviewed and re-issued periodically, the IRAC MoA minimised, and resistance is less likely to evolve. Sequence of acaricides through season classification list provides farmers, growers, advisors, extension staff, consultants and crop protection professionals witH a guide to the selection of Applications are often arranged into MoA spray windows or blocks that are defined by the stage of crop development and the biology of the pest species of concern. Local expert advice should acaricides and insecticides in resistance management programs. Effective always be followed witH regard to spray windows and timings. Several sprays may be possible witHin each spray window but it is generally essential to ensure that successive generations of the Resistance management of this type preserves the utility and diversity of pest are not treated witH compounds from the same MoA group. -

Guide to Ticks and Tick-Borne Diseases
Integrated Pest Management GUIDE TO TICKS AND TICK-BORNE DISEASES Plant Protection Programs College of Agriculture, Food and Natural Resources Published by University of Missouri Extension IPM1032 This publication is part of a series of integrated pest CONTENTS management (IPM) manuals prepared by the Plant Protection Programs of the University of Missouri. Topics INTRODUCTION TO TICKS . 3 covered in the series include an introduction to scouting, Morphology . 4 weed identification and management, plant diseases, and Identification . .6 insects of field and horticultural crops. These IPM manuals Life cycle . .7 are available from MU Extension at the following address: Behavior . 8 Distribution and ecology . 10 Extension Publications MEDICALLY IMPORTANT TICKS . .12 2800 Maguire Blvd. Lone star tick (Amblyomma americanum) . 12 Columbia, MO 65211 American dog tick (Dermacentor variabilis) .13 800-292-0969 Blacklegged tick (Ixodes scapularis) . 13 Brown dog tick (Rhipicephalus sanguineus) . 14 Relapsing fever tick (Ornithodoros turicata) 14 Bat tick (Ornithodoros kelleyi) . .15 Author Richard M. Houseman TICK-BORNE DISEASES . .16 Associate Professor of Entomology Human ehrlichiosis . 16 University of Missouri Extension Rocky Mountain spotted fever . 17 Southern tick-associated rash illness . .17 Lyme disease . 18. On the cover Anaplasmosis . 18 Dorsal view of a female lone star tick, Tick-borne relapsing fever . 19 Amblyomma americanum. Photo credit: James Tularemia . 19. Gathany, CDC INDIVIDUAL PERSONAL PROTECTION . 20 Photo credits Tick bite prevention . .20 Tick checks . 22 All photos were provided by the author, unless Tick removal . 22 otherwise indicated. Self-monitoring and medical treatment . 23 Follow-up . 24 Credits Centers for Disease Control and Prevention INTEGRATED PEST MANAGEMENT (IPM) (CDC) OF TICK POPULATIONS . -
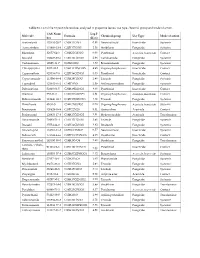
Table S1. List of the 33 Pesticide Residues Analyzed in Grapevine Leaves, Use Type, Chemical Group and Mode of Action. Molecule
Table S1. List of the 33 pesticide residues analyzed in grapevine leaves, use type, chemical group and mode of action. CAS Num‐ Log P Molecule Formula Chemical group Use Type Mode of action ber (KOW) Acetamiprid 135410‐20‐7 C10H11ClN4 0.80 Neonicotinoid Insecticide Systemic Azoxystrobin 131860‐33‐8 C22H17N3O5 2.50 Strobilurin Fungicide Systemic Bifenthrin 82657‐04‐3 C23H22ClF3O2 6.00 Pyrethroid Acaricide, Insecticide Contact Boscalid 188425‐85‐6 C18H12Cl2N2O 2.96 Carboxamide Fungicide Systemic Carbendazim 10605‐21‐7 C9H9N3O2 1.52 Benzimidazole Fungicide Systemic Chlorpyriphos 2921‐88‐2 C9H11Cl3NO3PS 4.96 Organophosphorous Insecticide Contact Cypermethrin 52315‐07‐8 C22H19Cl2NO3 5.55 Pyrethroid Insecticide Contact Cyproconazole 113096‐99‐4 C15H18ClN3O 2.90 Triazole Fungicide Systemic Cyprodinil 121552‐61‐2 C14H15N3 3.59 Anilinopyrimidine Fungicide Systemic Deltamethrin 52918‐63‐5 C22H19Br2NO3 4.60 Pyrethroid Insecticide Contact Diazinon 333‐41‐5 C12H21N2O3PS 3.81 Organophosphorous Acaricide, Insecticide Contact Difenoconazole 119446‐68‐3 C19H17Cl2N3O3 4.40 Triazole Fungicide Systemic Dimethoate 60‐51‐5 C5H12NO3PS2 0.78 Organophosphorous Acaricide, Insecticide Systemic Fenazaquin 120928‐09‐8 C20H22N2O 5.51 Quinazoline Acaricide Contact Fenhexamid 126833‐17‐8 C14H17Cl2NO2 3.51 Hydroxyanilide Acaricide Translaminar Hexaconazole 79983‐71‐4 C14H17Cl2N3O 3.90 Triazole Fungicide Systemic Imazalil 35554‐44‐0 C14H14Cl2N2O 3.82 Imidazole Fungicide Systemic Imidacloprid 138261‐41‐3 C9H10ClN5O2 0.57 Neonicotinoid Insecticide Systemic Indoxacarb 173584‐44‐6 -

Livestock Pest Management: a Training Manual for Commercial Pesticide Applicators (Category 1D)
Livestock Pest Management: A Training Manual for Commercial Pesticide Applicators (Category 1D) Edward D. Walker Julie A. Stachecki 1 Preface This manual is intended to prepare pesticide Materials from several sources were used to com- applicators in category 1D, livestock pest man- pile this information with input from North Car- agement, for certification under the Act 451, Nat- olina Extension, Key to Insect and Mites; Fly ural Resources and Environmental Protection Act, Control in Confined Livestock and Poultry Pro- Part 83, Pesticide Control, Sections 8301 to 8336. duction, Ciba-Geigy Agricultural Division, Read the introduction to this manual to under- Greensboro, NC; Managing Insect Problems on stand your responsibilities for obtaining the Beef Cattle, Kansas State University, Manhattan, appropriate credentials to apply pesticides and KS; Poultry Pest Management for Pennsylvania how to use this manual. and the Northeast, The Pennsylvania State Uni- versity, Extension Service, College of Agriculture, Acknowledgements University Park, PA; Pest Management Principles for the Commercial Applicator: Animal Pest Con- This manual, “Livestock Pest Management: A trol, University of Wisconsin Extension, Madison, Training Manual for Commercial Pesticide Appli- WI; and External Parasites of Poultry, Purdue cators (Category 1D),” was produced by Michi- University Extension, West Lafayette, IN, pho- gan State University, Pesticide Education tographs from Harlan Ritchie, Michigan State Program in conjunction with the Michigan University, East Lansing, MI, Ned Walker, Michi- Department of Agriculture. The following people gan State University, East Lansing, MI, Vocational are recognized for their reviews, suggestions and Education Publications, California Polytechnic contributions to this manual: State University San Luis Obispo, CA, Ralph Cle- George Atkeson, Ionia county agriculture agent, venger, Santa Rosa, CA, and Lepp and Associates, Michigan State University Extension, Ionia, MI Los Osis, CA. -

California Fish and Wildlife Journal, Special Issue 2, 2020, Pesticides in California
30 CALIFORNIA FISH AND WILDLIFE, CANNABIS SPECIAL ISSUE 2020 Great horned owl (Bubo virginianus) on an artificial perch at a cannabis cultivation in southern Humboldt County. Owls are an excellent natural rodent-control agent and providing perches and nest boxes for them can increase their presence on cultivation sites and reduce the need for rodenticides. Photo Credit: Ryan Mathis, CDFW California Fish and Wildlife, Cannabis Special Issue; 31-53; 2020 Pesticides in California: their potential impacts on wildlife resources and their use in permitted cannabis cultivation LINDSEY N. RICH1*, STELLA MCMILLIN2, ANGE DARNELL BAKER3, AND ERIN CHAPPELL4 1, 4California Department of Fish and Wildlife, Nongame Wildlife Program, 1010 Riverside Parkway, West Sacramento, CA 95605, USA 2 California Department of Fish and Wildlife, Wildlife Investigations Lab, 1701 Nimbus Road, Rancho Cordova, CA 95670 USA 3 California Department of Fish and Wildlife, Habitat Conservation and Planning Branch, 1010 Riverside Parkway, West Sacramento, CA 95605, USA *Corresponding Author: [email protected] The agricultural industry, including commercial cannabis cultivators, often relies on rodenticides and insecticides to help minimize damage from wildlife and insect pest species. Many of the most toxic pesticides are listed as California restricted materials, meaning they can only be purchased and used by certified applicators under a permit from a County Agricultural Commissioner. Despite the permit requirement and other restrictions, exposure of non-target wildlife to pesticides continues to occur throughout California. Non-target wildlife may be directly exposed through ingestion, inhalation, or dermal contact or second- arily exposed through ingestion of contaminated or poisoned prey. Exposure to pesticides can be lethal, or it can cause sublethal effects that impact species’ immunology, reproduction, thermoregulation, morphology, and behavior. -

Acaricide (Chemical) Resistance in Cattle Ticks Introduction
Fact sheet Acaricide (chemical) resistance in cattle ticks Introduction Cattle ticks have the ability to adapt over time so that they, and their offspring, build up resistance to tick treatments. The prolonged or incorrect use of tick chemicals can lead to resistance in ticks. Resistance enables the ticks to tolerate and survive chemical applications. Pesticides that kill mites and ticks are referred to as ‘acaricides’, which is why this form of resistance is called acaricide (chemical) resistance. An increase in chemical resistance in cattle ticks could result in current routine tick treatments becoming ineffective, making tick control in the future much more difficult. It is important to preserve the chemicals available by restricting the spread of resistant ticks throughout the Northern Territory (NT). This can be achieved by adhering to movement requirements relating to cattle ticks, the correct use of chemicals and good dip management. Acaricide chemical groups A number of chemical groups are available for controlling cattle ticks in Australia. Resistance depends on the chemical group used and its rate of application over time. There have been some cases of acaricide resistance to the Macrocylic Lactone (ML) group in Queensland, which include ‘mectin’-based chemicals, such as ivermectin. Table 1. Chemical groups of acaricides used for controlling cattle ticks and the associated resistant strains Resistant strains of cattle ticks (Rhipicephalus microplus) Chemical groups Biarra Mt Ulam Lamington Parkhurst Ultimo Alford Organophosphates (OP) ✕ ✓ ✓ ✕ ✕ Synthetic pyrethroids ✓ ✓ ✕ ✕ ✕ (SP) Amitraz ✓ ✕ ✓ ✓ ✕ Macrocyclic lactone ✓ ✓ ✓ ✓ ✓ (ML) Department of Primary Industry and Resources Page 1 of 4 Acaricide (chemical) resistance in cattle ticks Acaricide resistance in Australia and the Northern Territory Queensland, New South Wales and the Northern Territory (NT) all have properties with acaricide resistant cattle ticks. -

Insecticide/Acaricide Resistance in Fleas and Ticks Infesting Dogs and Cats Tad B Coles1* and Michael W Dryden2
Coles and Dryden Parasites & Vectors 2014, 7:8 http://www.parasitesandvectors.com/content/7/1/8 REVIEW Open Access Insecticide/acaricide resistance in fleas and ticks infesting dogs and cats Tad B Coles1* and Michael W Dryden2 Abstract This review defines insecticide/acaricide resistance and describes the history, evolution, types, mechanisms, and detection of resistance as it applies to chemicals currently used against fleas and ticks of dogs and cats and summarizes resistance reported to date. We introduce the concept of refugia as it applies to flea and tick resistance and discuss strategies to minimize the impact and inevitable onset of resistance to newer classes of insecticides. Our purpose is to provide the veterinary practitioner with information needed to investigate suspected lack of efficacy, respond to lack of efficacy complaints from their clients, and evaluate the relative importance of resistance as they strive to relieve their patients and satisfy their clients when faced with flea and tick infestations that are difficult to resolve. We conclude that causality of suspected lack of insecticide/acaricide efficacy is most likely treatment deficiency, not resistance. Keywords: Flea, Ctenocephalides felis, Tick, Rhipicephalus sanguineus, Dog, Cat, Insecticide, Acaricide, Resistance, Refugia, Refugium Review nuisance pests of dogs, cats, and their human owners: Background Ctenocephalides felis felis (cat flea), C. canis (dog flea), In this paper we will review the current information relative Echidnophaga gallinacea (sticktight flea), Pulex irritans to resistance of fleas and ticks to insecticides and acaricides, (human flea), and the closely related P. simulans [2-4]. respectively, as it applies to canine and feline veterinary Ctenocephalides felis is by far the most common flea practitioners. -

Recognition and Management of Pesticide Poisonings: Sixth Edition: 2013: Chapter 9 Other Insecticides
HIGHLIGHTS CHAPTER 9 Multiple agents with widely varying toxicity Agents of concern Other Insecticides and Acaricides include borates, fluorides, pyrethroids Neonicotinoids are a newer class that merits attention This chapter concerns insecticides and acaricides having toxicologic characteris- due to widespread use and tics distinct from the insecticides discussed in previous chapters. It discusses benzyl toxicity benzoate, borates, chlordimeform, chlorobenzilate, cyhexatin, fluorides, fipronil (an n-phenylpyrazone insecticide), haloaromatic substituted urea compounds, metho- prene, neonicotinoids, propargite and sulfur. SIGNS & SYMPTOMS Variable and highly related BENZYL BENZOATE to the specific agent Incorporated into lotions and ointments, this agent has been used for many years in Boric acid, fluorides, veterinary and human medicine against mites and lice. n-phenylpyrazones and neonicotinoids should be suspected in cases with Toxicology CNS symptoms Apart from occasional cases of skin irritation, adverse effects have been few. The effi- ciency of skin absorption is not known. Absorbed benzyl benzoate is rapidly biotrans- formed to hippuric acid that is excreted in the urine. Oral toxicity in animals is low, TREATMENT with LD50 values in the 2-3 grams/kg range in rats and cats. When given in large doses to laboratory animals, benzyl benzoate causes excitement, incoordination, paralysis of Specific to agent the limbs, convulsions, respiratory paralysis and death.1 Very few human exposures Skin/eye decontamination have been reported to the National Poison Data System. Consider GI decontamination based on Treatment quantity and time interval factors 1. If significant irritant effect appears, discontinue use of product and cleanse skin with soap and water. Treat eye contamination by irrigating exposed eyes with Severe CNS symptoms copious amounts of clean water or saline for at least 15 minutes. -

Banned Pesticide List
BANNED PESTICIDE tIST No Common Name Trade Name Pesticide Type Toxicity Class 1 1,1,2,2-tetra chloroethane Acetylene tetrachloride (rum eanr) Insecticide lb 2 aldrin Octaiene, Aldrex Insecticide Ib 3 AZN Insecticide Ib azinphos'methyl Gusathion Insecticide Ib 5 Insecti€ide Ib 6 camphechlor Insecticide Ib I chlordane Octarhior Insecticide II 8 Insecticide Ib 9 nsecticide IPA I 10 chlormephos Dotan Insecticide Ia 11 Insecticide Ia 12 DDI Anofex, Neocide, Chlorophenthoate Insecticide Ib jeldrer, dieldrin Octalor, d dieldrite Insectrcrde Ia 1.4 endosulfan Thiodan (veryh shlvroxicto lish) lnsecticide ll Endocide lnsecticide Ib endrin Hexadrin, Endrix, Mendrin lnsecticide Ia 1l ethylene dichloride Bualta, Eusan 77 Insecticide I 18 ethylene oxide Oxirane {iumieano lnsecticide In 19 flucythrinate Cybolt, Cythrin, Pay off Insecticide Ib 20 D!,fonate lnse€ticide Ia 21 Deltanet, Promet Insecticide Ib 22 gamma-HCH (hexachlorocyctohexdne) Gamma-Col, Lindane Insecticide II 23 heptachlor Heptamul, Heptox, Hepta lnsecticide II )4 heptenophos Hostaquick, Ragadan lnsectlcrde 2.,.,':'.t )X Ib 25 isophenphos Oftanol, Insecticide /.,j'/ tE' \: tb 26 isoxathion Karphos, rnsecrcrde .t.l L r&'(l) l.\ Ib 27 Jumbda cyhalothrin Karate (very hishlyroxic to fish) Insecr,c'de Ui\ €,,/./' II 2A methylene chlofide Dichloromethane (rumisan0 Insecticide \r --\>:7 */ II 29 Mirex lnsecticide Ib 30 nicotine Nico SoaD Insecticide Ib oxvdemeton-methvl Metasvstox R, lnsecticide Ib 32 parathion-methyl Foldol, Metacide, Fostox lnsecticide I 33 pirimiphos-ethyl Primicid lnsecticide