Guide to Ticks and Tick-Borne Diseases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluating the Risk of Tick-Borne Relapsing Fever Among Occupational Cavers—Austin, TX, 2017
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Zoonoses Manuscript Author Public Health Manuscript . Author Author manuscript; available in PMC 2020 February 26. Published in final edited form as: Zoonoses Public Health. 2019 September ; 66(6): 579–586. doi:10.1111/zph.12588. Evaluating the risk of tick-borne relapsing fever among occupational cavers—Austin, TX, 2017 Stefanie B. Campbell1, Anna Klioueva2, Jeff Taylor2, Christina Nelson1, Suzanne Tomasi3, Adam Replogle1, Natalie Kwit1, Christopher Sexton1, Amy Schwartz1, Alison Hinckley1 1Centers for Disease Control and Prevention, Fort Collins, Colorado 2Austin Public Health, Austin, Texas 3Centers for Disease Control and Prevention, Morgantown, West Virginia Abstract Tick-borne relapsing fever (TBRF) is a potentially serious spirochetal infection caused by certain species of Borrelia and acquired through the bite of Ornithodoros ticks. In 2017, Austin Public Health, Austin, TX, identified five cases of febrile illness among employees who worked in caves. A cross-sectional serosurvey and interview were conducted for 44 employees at eight organizations that conduct cave-related work. Antibodies against TBRF-causing Borrelia were detected in the serum of five participants, four of whom reported recent illness. Seropositive employees entered significantly more caves (Median 25 [SD: 15] versus Median 4 [SD: 16], p = 0.04) than seronegative employees. Six caves were entered more frequently by seropositive employees posing a potentially high risk. Several of these caves were in public use areas and were opened for tours. Education of area healthcare providers about TBRF and prevention recommendations for cavers and the public are advised. Keywords Borrelia; Borrelia hermsii; Borrelia turicatae; TBRF; TX 1 | INTRODUCTION Tick-borne relapsing fever (TBRF) in humans follows infection with one of several Borrelia bacteria species. -

Information to Users
INFORMATION TO USERS The most advanced technology has been used to photograph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6" x 9" black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. University Microfilms International A Bell & Howell Information Company 300 North Zeeb Road. Ann Arbor, Ml 48106-1346 USA 313/761-4700 800/521-0600 Order Number 9111799 Evolutionary morphology of the locomotor apparatus in Arachnida Shultz, Jeffrey Walden, Ph.D. -

INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES
US Environmental Protection Agency Office of Pesticide Programs INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES Note: Pesticide tolerance information is updated in the Code of Federal Regulations on a weekly basis. EPA plans to update these indexes biannually. These indexes are current as of the date indicated in the pdf file. For the latest information on pesticide tolerances, please check the electronic Code of Federal Regulations (eCFR) at http://www.access.gpo.gov/nara/cfr/waisidx_07/40cfrv23_07.html 1 40 CFR Type Family Common name CAS Number PC code 180.163 Acaricide bridged diphenyl Dicofol (1,1-Bis(chlorophenyl)-2,2,2-trichloroethanol) 115-32-2 10501 180.198 Acaricide phosphonate Trichlorfon 52-68-6 57901 180.259 Acaricide sulfite ester Propargite 2312-35-8 97601 180.446 Acaricide tetrazine Clofentezine 74115-24-5 125501 180.448 Acaricide thiazolidine Hexythiazox 78587-05-0 128849 180.517 Acaricide phenylpyrazole Fipronil 120068-37-3 129121 180.566 Acaricide pyrazole Fenpyroximate 134098-61-6 129131 180.572 Acaricide carbazate Bifenazate 149877-41-8 586 180.593 Acaricide unclassified Etoxazole 153233-91-1 107091 180.599 Acaricide unclassified Acequinocyl 57960-19-7 6329 180.341 Acaricide, fungicide dinitrophenol Dinocap (2, 4-Dinitro-6-octylphenyl crotonate and 2,6-dinitro-4- 39300-45-3 36001 octylphenyl crotonate} 180.111 Acaricide, insecticide organophosphorus Malathion 121-75-5 57701 180.182 Acaricide, insecticide cyclodiene Endosulfan 115-29-7 79401 -

Tick-Borne Relapsing Fever CLAY ROSCOE, M.D., and TED EPPERLY, M.D., Family Medicine Residency of Idaho, Boise, Idaho
Tick-Borne Relapsing Fever CLAY ROSCOE, M.D., and TED EPPERLY, M.D., Family Medicine Residency of Idaho, Boise, Idaho Tick-borne relapsing fever is characterized by recurring fevers separated by afebrile periods and is accompanied by nonspecific constitutional symptoms. It occurs after a patient has been bitten by a tick infected with a Borrelia spirochete. The diagnosis of tick-borne relapsing fever requires an accurate characterization of the fever and a thorough medical, social, and travel history of the patient. Findings on physical examination are variable; abdominal pain, vomiting, and altered sensorium are the most common symptoms. Laboratory confirmation of tick-borne relapsing fever is made by detection of spirochetes in thin or thick blood smears obtained during a febrile episode. Treatment with a tetracycline or macrolide antibiotic is effective, and antibiotic resistance is rare. Patients treated for tick-borne relapsing fever should be monitored closely for Jarisch- Herxheimer reactions. Fatalities from tick-borne relapsing fever are rare in treated patients, as are subsequent Jarisch-Herxheimer reactions. Persons in endemic regions should avoid rodent- and tick-infested areas and use insect repellents and protective clothing to prevent tick bites. (Am Fam Physician 2005;72:2039-44, 2046. Copyright © 2005 American Academy of Family Physicians.) S Patient information: ick-borne relapsing fever (TBRF) develop with TBRF, with long-term sequelae A handout on tick-borne is transmitted by Ornithodoros that may be permanent. Reviewing a broad relapsing fever, written by 1,3-6 the authors of this article, ticks infected with one of sev- differential diagnosis (Table 1 ) for fever is provided on page 2046. -

Best Management Practices for Brown Dog Ticks
Best Management Practices For Brown Dog Ticks immediately seek shelter in cracks or crevices such as baseboards or furniture, but also commonly move en masse up walls and congregate in the corners of ceilings. These larvae eventually find the dog and receive their first blood meal. The larvae go largely unnoticed because they are about the size of a pencil tip. The larvae then drop off into the surrounding area, molt to the nymphal stage, and again seek the dog for a second blood meal. At this point owners occasionally notice the ticks, but they usually go unnoticed. After they molt to the adult stage, the ticks find the dog for a third and final blood meal. Ticks are noticed at this stage for two reasons: 1) Adult females engorge to the size of a raisin and are often located on or near the dog’s head, or 2) adults are seen Heavy infestation of BDT on the ear of a dog. crawling on floors actively looking for the dog. This “predatory” behavior is somewhat unique to ticks. The brown dog tick (BDT) can be a serious Typically, residents do not notice these ticks until they have pest in homes with pets. These Best Management completed a full generation. Often, overlapping generations of Practices are designed to support cooperation ticks occur in homes, so tick numbers can quickly multiply into the thousands. between homeowners and pest management A factor complicating BDT management is the ability of this professionals in order to prevent and control BDTs. tick to survive without a host for several months during each of At left: The black-legged its three life stages, thereby negating the “wait-it-out” strategy of tick (Ixodes scapularis). -

Sound Management of Pesticides and Diagnosis and Treatment Of
* Revision of the“IPCS - Multilevel Course on the Safe Use of Pesticides and on the Diagnosis and Treatment of Presticide Poisoning, 1994” © World Health Organization 2006 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. CONTENTS Preface Acknowledgement Part I. Overview 1. Introduction 1.1 Background 1.2 Objectives 2. Overview of the resource tool 2.1 Moduledescription 2.2 Training levels 2.3 Visual aids 2.4 Informationsources 3. Using the resource tool 3.1 Introduction 3.2 Training trainers 3.2.1 Organizational aspects 3.2.2 Coordinator’s preparation 3.2.3 Selection of participants 3.2.4 Before training trainers 3.2.5 Specimen module 3.3 Trainers 3.3.1 Trainer preparation 3.3.2 Selection of participants 3.3.3 Organizational aspects 3.3.4 Before a course 4. -

Acaricide Mode of Action Classification: a Key to Effective Acaricide Resistance Management Insecticide Resistance Action Committee
Acaricide Mode of Action Classification: A key to effective acaricide resistance management Insecticide Resistance Action Committee www.irac-online.org Introduction Effective IRM strategies: Sequences or alternations of MoA IRAC promotes the use of a Mode of Action (MoA) classification of All effective pesticide resistance management strategies seek to minimise the selection of resistance to any one type of MoA w MoA x MoA y MoA z MoA w MoA x insecticides and acaricides as the basis for effective and sustainable pesticide. In practice, alternations, sequences or rotations of compounds from different MoA groups provide sustainable and resistance management. Acaricides are allocated to specific groups based effective resistance management for acarine pests. This ensures that selection from compounds in the same MoA group is on their target site. Reviewed and re-issued periodically, the IRAC MoA minimised, and resistance is less likely to evolve. Sequence of acaricides through season classification list provides farmers, growers, advisors, extension staff, consultants and crop protection professionals witH a guide to the selection of Applications are often arranged into MoA spray windows or blocks that are defined by the stage of crop development and the biology of the pest species of concern. Local expert advice should acaricides and insecticides in resistance management programs. Effective always be followed witH regard to spray windows and timings. Several sprays may be possible witHin each spray window but it is generally essential to ensure that successive generations of the Resistance management of this type preserves the utility and diversity of pest are not treated witH compounds from the same MoA group. -

Physiological Resistance Alters Behavioral Response of Tetranychus Urticae to Acaricides Adekunle W
www.nature.com/scientificreports OPEN Physiological resistance alters behavioral response of Tetranychus urticae to acaricides Adekunle W. Adesanya1,2*, Michael J. Beauchamp1, Mark D. Lavine2, Laura C. Lavine2, Fang Zhu 2,3 & Doug B. Walsh1,2 Multiple acaricide resistance in Tetranychus urticae continues to threaten crop production globally, justifying the need to adequately study resistance for sustainable pest management. Most studies on acaricide resistance have focused on the acute contact toxicity of acaricides with little or no information on the behavioral responses elicited after acaricide exposure. Furthermore, the impact of physiological resistance on these behavioral responses remains unknown in most pest species, including T. urticae. We tested the efect of acaricide resistance on contact toxicity, irritancy and repellency of mitochondrial electron transport inhibitor of complex I (MET-I) and mite growth inhibitor (MGI) acaricides on multiple T. urticae strains. We also tested whether acaricides with similar physiological target site/mode of action also elicit similar behavioral efects on T. urticae strains. MET-I acaricides (fenazaquin, fenpyroximate, and pyrabiden) and MGIs (clofentezine, hexythiazox and etoxazole) elicited a dose-dependent irritant and repellent efect on T. urticae. Selection of strains for physiological resistance to these acaricides afected the behavioral response of T. urticae, especially in MET-I resistant strains, that showed reduced irritancy and repellency to MET-I acaricides. Behavioral response also afected the oviposition of T. urticae, where strains generally showed preferential oviposition away from the acaricides. The outcome of this study highlights negative consequences of acaricide resistance that can potentially afect T. urticae management. In addition to a pesticide’s direct lethality, its sublethal efects can also afect its efcacy1. -
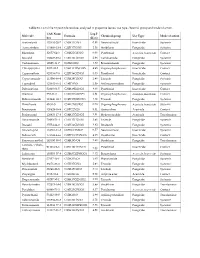
Table S1. List of the 33 Pesticide Residues Analyzed in Grapevine Leaves, Use Type, Chemical Group and Mode of Action. Molecule
Table S1. List of the 33 pesticide residues analyzed in grapevine leaves, use type, chemical group and mode of action. CAS Num‐ Log P Molecule Formula Chemical group Use Type Mode of action ber (KOW) Acetamiprid 135410‐20‐7 C10H11ClN4 0.80 Neonicotinoid Insecticide Systemic Azoxystrobin 131860‐33‐8 C22H17N3O5 2.50 Strobilurin Fungicide Systemic Bifenthrin 82657‐04‐3 C23H22ClF3O2 6.00 Pyrethroid Acaricide, Insecticide Contact Boscalid 188425‐85‐6 C18H12Cl2N2O 2.96 Carboxamide Fungicide Systemic Carbendazim 10605‐21‐7 C9H9N3O2 1.52 Benzimidazole Fungicide Systemic Chlorpyriphos 2921‐88‐2 C9H11Cl3NO3PS 4.96 Organophosphorous Insecticide Contact Cypermethrin 52315‐07‐8 C22H19Cl2NO3 5.55 Pyrethroid Insecticide Contact Cyproconazole 113096‐99‐4 C15H18ClN3O 2.90 Triazole Fungicide Systemic Cyprodinil 121552‐61‐2 C14H15N3 3.59 Anilinopyrimidine Fungicide Systemic Deltamethrin 52918‐63‐5 C22H19Br2NO3 4.60 Pyrethroid Insecticide Contact Diazinon 333‐41‐5 C12H21N2O3PS 3.81 Organophosphorous Acaricide, Insecticide Contact Difenoconazole 119446‐68‐3 C19H17Cl2N3O3 4.40 Triazole Fungicide Systemic Dimethoate 60‐51‐5 C5H12NO3PS2 0.78 Organophosphorous Acaricide, Insecticide Systemic Fenazaquin 120928‐09‐8 C20H22N2O 5.51 Quinazoline Acaricide Contact Fenhexamid 126833‐17‐8 C14H17Cl2NO2 3.51 Hydroxyanilide Acaricide Translaminar Hexaconazole 79983‐71‐4 C14H17Cl2N3O 3.90 Triazole Fungicide Systemic Imazalil 35554‐44‐0 C14H14Cl2N2O 3.82 Imidazole Fungicide Systemic Imidacloprid 138261‐41‐3 C9H10ClN5O2 0.57 Neonicotinoid Insecticide Systemic Indoxacarb 173584‐44‐6 -

MORFOLOGIA E DESENVOLVIMENTO DO SISTEMA REPRODUTOR MASCULINO DE CARRAPATOS DO GÊNERO Amblyomma (ACARI, IXODIDAE): UMA ANÁLISE COMPARATIVA
UNIVERSIDADE ESTADUAL PAULISTA “JÚLIO DE MESQUITA FILHO” INSTITUTO DE BIOCIÊNCIAS – RIO unesp CLARO PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS BIOLÓGICAS (BIOLOGIA CELULAR E MOLECULAR) MORFOLOGIA E DESENVOLVIMENTO DO SISTEMA REPRODUTOR MASCULINO DE CARRAPATOS DO GÊNERO Amblyomma (ACARI, IXODIDAE): UMA ANÁLISE COMPARATIVA BRUNO RODRIGUES SAMPIERI Tese apresentada ao Instituto de Biociências do Campus de Rio Claro, Universidade Estadual Paulista, como parte dos requisitos para obtenção do título de Doutor em Ciências Biológicas (Biologia Celular e Molecular). Maio - 2016 UNIVERSIDADE ESTADUAL PAULISTA “JÚLIO DE MESQUITA FILHO” INSTITUTO DE BIOCIÊNCIAS – RIO unesp CLARO PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS BIOLÓGICAS (BIOLOGIA CELULAR E MOLECULAR) MORFOLOGIA E DESENVOLVIMENTO DO SISTEMA REPRODUTOR MASCULINO DE CARRAPATOS DO GÊNERO Amblyomma (ACARI, IXODIDAE): UMA ANÁLISE COMPARATIVA BRUNO RODRIGUES SAMPIERI ORIENTADORA: PROFA. DRA. MARIA IZABEL CAMARGO-MATHIAS CO-ORIENTADOR: PROF. DR. ODAIR CORREA BUENO Tese apresentada ao Instituto de Biociências do Campus de Rio Claro, Universidade Estadual Paulista, como parte dos requisitos para obtenção do título de Doutor em Ciências Biológicas (Biologia Celular e Molecular). Maio - 2016 591.4 Sampieri, Bruno Rodrigues S192m Morfologia e desenvolvimento do sistema reprodutor masculino de carrapatos do gênero Amblyomma (Acari, Ixodidae) : uma análise comparativa / Bruno Rodrigues Sampieri. - Rio Claro, 2016 113 f. : il., figs., tabs. Tese (doutorado) - Universidade Estadual Paulista, Instituto de Biociências de Rio Claro Orientadora: Maria Izabel Souza Camargo Coorientador: Odair Correa Bueno 1. Anatomia comparada. 2. Espermiotaxonomia. 3. Espermiocladística. 4. Filogenia. 5. Controle. I. Título. Ficha Catalográfica elaborada pela STATI - Biblioteca da UNESP Campus de Rio Claro/SP Dedicatória Dedico esta tese aos meus pais, à minha esposa, à minha filha e ao meu irmão, pelo apoio, companheirismo e paciência; por compartilharem comigo angústias e conquistas. -

Pathogen and Host Response Dynamics in a Mouse Model of Borrelia Hermsii Relapsing Fever
veterinary sciences Article Pathogen and Host Response Dynamics in a Mouse Model of Borrelia hermsii Relapsing Fever Christopher D. Crowder, Arash Ghalyanchi Langeroudi, Azadeh Shojaee Estabragh, Eric R. G. Lewis, Renee A. Marcsisin and Alan G. Barbour * Departments of Microbiology & Molecular Genetics and Medicine, University of California Irvine, Irvine, CA 92697, USA; [email protected] (C.D.C.); [email protected] (A.G.L.); [email protected] (A.S.E.); [email protected] (E.R.G.L.); [email protected] (R.A.M.) * Correspondence: [email protected]; Tel.: +1-949-824-5626 Academic Editor: Ulrike Munderloh Received: 13 July 2016; Accepted: 24 August 2016; Published: 30 August 2016 Abstract: Most Borrelia species that cause tick-borne relapsing fever utilize rodents as their natural reservoirs, and for decades laboratory-bred rodents have served as informative experimental models for the disease. However, while there has much progress in understanding the pathogenetic mechanisms, including antigenic variation, of the pathogen, the host side of the equation has been neglected. Using different approaches, we studied, in immunocompetent inbred mice, the dynamics of infection with and host responses to North American relapsing fever agent B. hermsii. The spirochete’s generation time in blood of infected mice was between 4–5 h and, after a delay, was matched in rate by the increase of specific agglutinating antibodies in response to the infection. After initiating serotype cells were cleared by antibodies, the surviving spirochetes were a different serotype and, as a population, grew more slowly. The retardation was attributable to the host response and not an inherently slower growth rate. -

Livestock Pest Management: a Training Manual for Commercial Pesticide Applicators (Category 1D)
Livestock Pest Management: A Training Manual for Commercial Pesticide Applicators (Category 1D) Edward D. Walker Julie A. Stachecki 1 Preface This manual is intended to prepare pesticide Materials from several sources were used to com- applicators in category 1D, livestock pest man- pile this information with input from North Car- agement, for certification under the Act 451, Nat- olina Extension, Key to Insect and Mites; Fly ural Resources and Environmental Protection Act, Control in Confined Livestock and Poultry Pro- Part 83, Pesticide Control, Sections 8301 to 8336. duction, Ciba-Geigy Agricultural Division, Read the introduction to this manual to under- Greensboro, NC; Managing Insect Problems on stand your responsibilities for obtaining the Beef Cattle, Kansas State University, Manhattan, appropriate credentials to apply pesticides and KS; Poultry Pest Management for Pennsylvania how to use this manual. and the Northeast, The Pennsylvania State Uni- versity, Extension Service, College of Agriculture, Acknowledgements University Park, PA; Pest Management Principles for the Commercial Applicator: Animal Pest Con- This manual, “Livestock Pest Management: A trol, University of Wisconsin Extension, Madison, Training Manual for Commercial Pesticide Appli- WI; and External Parasites of Poultry, Purdue cators (Category 1D),” was produced by Michi- University Extension, West Lafayette, IN, pho- gan State University, Pesticide Education tographs from Harlan Ritchie, Michigan State Program in conjunction with the Michigan University, East Lansing, MI, Ned Walker, Michi- Department of Agriculture. The following people gan State University, East Lansing, MI, Vocational are recognized for their reviews, suggestions and Education Publications, California Polytechnic contributions to this manual: State University San Luis Obispo, CA, Ralph Cle- George Atkeson, Ionia county agriculture agent, venger, Santa Rosa, CA, and Lepp and Associates, Michigan State University Extension, Ionia, MI Los Osis, CA.