Recognition and Management of Pesticide Poisonings: Sixth Edition: 2013: Chapter 9 Other Insecticides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Insecticide Use for Vector-Borne Disease Control
WHO/CDS/NTD/WHOPES/GCDPP/2007.2 GLOBAL INSECTICIDE USE FOR VECTOR-BORNE DISEASE CONTROL M. Zaim & P. Jambulingam DEPARTMENT OF CONTROL OF NEGLECTED TROPICAL DISEASES (NTD) WHO PESTICIDE EVALUATION SCHEME (WHOPES) First edition, 2002 Second edition, 2004 Third edition, 2007 © World Health Organization 2007 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. The named authors alone are responsible for the views expressed in this publication. CONTENTS Page Acknowledgements i Introduction 1 Collection of information 2 Data analysis and observations on reporting 3 All uses in vector control 6 Malaria vector control 22 Dengue vector control 38 Chagas disease vector control 48 Leishmaniasis vector control 52 Other vector-borne disease control 56 Selected insecticides – DDT 58 Selected insecticides – Insect growth regulators 60 Selected insecticides – Bacterial larvicides 62 Country examples 64 Annex 1. -
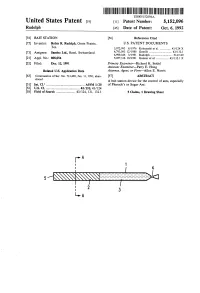
United States Patent (19) 11 Patent Number: 5,152,096 Rudolph 45 Date of Patent: Oct
III USOO5152096A United States Patent (19) 11 Patent Number: 5,152,096 Rudolph 45 Date of Patent: Oct. 6, 1992 (54) BAIT STATION (56) References Cited (75) Inventor: Robin R. Rudolph, Grain Prairie, U.S. PATENT DOCUMENTS Tex. 3,972,993 8/1976 Kobayashi et al................ 43/124 X 4,793,093 12/1988 Gentile ............................... 43/132.1 (73) Assignee: Sandoz Ltd., Basel, Switzerland 4,999,346 3/1991 Rudolph .............................. 514/120 21 Appl. No.: 808,054 5,057,316 10/1991 Gunner et al. ................. 43/132.1 X 22 Filed: Dec. 12, 1991 Primary Examiner-Richard K. Seidel Assistant Examiner-Patty E. Hong Related U.S. Application Data Attorney, Agent, or Firm-Allen E. Norris 63 Continuation of Ser. No. 713,480, Jun. 11, 1991, aban 57) ABSTRACT doned. A bait station device for the control of ants, especially (51) Int. Cl. ............................................... A0M 1/20 of Pharaoh's or Sugar Ant. (52 U.S. C. ......................................... 43/131; 43/124 58) Field of Search ....................... 43/124, 131, 132.1 5 Claims, 1 Drawing Sheet U.S. Patent Oct. 6, 1992 5,152,096 DSN a- 5,152,096 1. 2 enting the ants with a combination of an insect growth BAIT STATION regulant (IGR) bait and insecticide bait in such a way that the worker ants have to forage their way through This is a continuation of application Ser. No. the IGR bait to reach the insecticide bait. 07/713,480, filed Jun. 11, 1991, now abandoned. 5 In this way foraging worker ants will transport back The present invention concerns a bait station device to nests for feeding of the colony IGR bait and upon for the control of ants, especially of Pharaoh's or Sugar exhausting the available IGR bait will themselves ingest Ant. -

Insecticide Residue Analyses in Cucumbers Sampled from Çanakkale Open Markets1 Çanakkale Açık Pazarlarından Örneklenen Hıyarlarda Insektisit Kalıntı Analizleri
Türk. entomol. derg., 2020, 44 (4): 449-460 ISSN 1010-6960 DOI: http://dx.doi.org/10.16970/entoted.767482 E-ISSN 2536-491X Original article (Orijinal araştırma) Insecticide residue analyses in cucumbers sampled from Çanakkale open markets1 Çanakkale açık pazarlarından örneklenen hıyarlarda insektisit kalıntı analizleri Hayriye ÇATAK2 Osman TİRYAKİ3* Abstract The aim of this study was to investigate four insecticide residues in cucumbers with the aid of QuEChERS 2007.1 method. For method verification assessment, pesticide-free cucumber matrix was spiked with 0.1, 1 and 10 times of MRL for each pesticide. The QuEChERS-LC-MS/MS analytical method revealed that the detection limits of the insecticides were below the MRLs and the overall recovery of method was 97.7%. These figures were within the SANTE recovery limits (60-140%) and the values specified for the repeatability (≤20%). Cucumbers were collected from six different stands (A-F) at Çanakkale open markets for 6 weeks between 23 November and 28 December 2018. Residues of each sampling time and each stand were assessed. Acetamiprid residue of 257g and 236 µg/kg were detected in week 5 from stand B and in week 2 from stand E, respectively. These values are close to MRL (300 µg/kg). Formetanate hydrochloride residue of the week 3 from stand F (36.3 µg/kg) was more than MRL of 10 µg/kg. Pirimiphos methyl and chlorpyrifos residues were not detected in cucumbers. Theoretical maximum daily intake assessment showed that there was no chronic exposure risk for these four pesticides through cucumber consumption. Keywords: Cucumber, insecticide residues, QuEChERS, risk assessment, toxicology Öz Bu çalışma hıyarlarda dört insektisit kalıntısını QuEChERS 2007.1 yöntemi ile belirlemek amacıyla yapılmıştır. -

Monitoring of Pesticide Residues in Commonly Used Fruits and Vegetables in Kuwait
International Journal of Environmental Research and Public Health Article Monitoring of Pesticide Residues in Commonly Used Fruits and Vegetables in Kuwait Mustapha F. A. Jallow *, Dawood G. Awadh, Mohammed S. Albaho, Vimala Y. Devi and Nisar Ahmad Environment and Life Sciences Research Center, Kuwait Institute for Scientific Research, P.O. Box 24885, Safat 13109, Kuwait; [email protected] (D.G.A.); [email protected] (M.S.A.); [email protected] (V.Y.D.); [email protected] (N.A.) * Correspondence: [email protected]; Tel.: +965-249-8984 Received: 1 May 2017; Accepted: 12 July 2017; Published: 25 July 2017 Abstract: The presence of pesticide residues in primary and derived agricultural products raises serious health concerns for consumers. The aim of this study was to assess the level of pesticide residues in commonly consumed fruits and vegetables in Kuwait. A total of 150 samples of different fresh vegetables and fruits were analyzed for the presence of 34 pesticides using the quick easy cheap effective rugged and safe (QuEChERS) multi-residue extraction, followed by gas chromatography-mass spectrometry (GC-MS) or liquid chromatography-tandem mass spectrometry (LC-MS/MS). Pesticide residues above the maximum residue limits (MRL) were detected in 21% of the samples and 79% of the samples had no residues of the pesticides surveyed or contained residues below the MRL. Multiple residues were present in 40% of the samples with two to four pesticides, and four samples were contaminated with more than four pesticide residues. Of the pesticides investigated, 16 were detected, of which imidacloprid, deltamethrin, cypermethrin, malathion, acetamiprid, monocrotophos, chlorpyrifos-methyl, and diazinon exceeded their MRLs. -

Ri Wkh% Lrorjlfdo (Iihfwv Ri 6Hohfwhg &Rqvwlwxhqwv
Guidelines for Interpretation of the Biological Effects of Selected Constituents in Biota, Water, and Sediment November 1998 NIATIONAL RRIGATION WQATER UALITY P ROGRAM INFORMATION REPORT No. 3 United States Department of the Interior Bureau of Reclamation Fish and Wildlife Service Geological Survey Bureau of Indian Affairs 8QLWHG6WDWHV'HSDUWPHQWRI WKH,QWHULRU 1DWLRQDO,UULJDWLRQ:DWHU 4XDOLW\3URJUDP LQIRUPDWLRQUHSRUWQR *XLGHOLQHVIRU,QWHUSUHWDWLRQ RIWKH%LRORJLFDO(IIHFWVRI 6HOHFWHG&RQVWLWXHQWVLQ %LRWD:DWHUDQG6HGLPHQW 3DUWLFLSDWLQJ$JHQFLHV %XUHDXRI5HFODPDWLRQ 86)LVKDQG:LOGOLIH6HUYLFH 86*HRORJLFDO6XUYH\ %XUHDXRI,QGLDQ$IIDLUV 1RYHPEHU 81,7('67$7(6'(3$570(172)7+(,17(5,25 %58&(%$%%,776HFUHWDU\ $Q\XVHRIILUPWUDGHRUEUDQGQDPHVLQWKLVUHSRUWLVIRU LGHQWLILFDWLRQSXUSRVHVRQO\DQGGRHVQRWFRQVWLWXWHHQGRUVHPHQW E\WKH1DWLRQDO,UULJDWLRQ:DWHU4XDOLW\3URJUDP 7RUHTXHVWFRSLHVRIWKLVUHSRUWRUDGGLWLRQDOLQIRUPDWLRQFRQWDFW 0DQDJHU1,:43 ' %XUHDXRI5HFODPDWLRQ 32%R[ 'HQYHU&2 2UYLVLWWKH1,:43ZHEVLWHDW KWWSZZZXVEUJRYQLZTS Introduction The guidelines, criteria, and other information in The Limitations of This Volume this volume were originally compiled for use by personnel conducting studies for the It is important to note five limitations on the Department of the Interior's National Irrigation material presented here: Water Quality Program (NIWQP). The purpose of these studies is to identify and address (1) Out of the hundreds of substances known irrigation-induced water quality and to affect wetlands and water bodies, this contamination problems associated with any of volume focuses on only nine constituents or the Department's water projects in the Western properties commonly identified during States. When NIWQP scientists submit NIWQP studies in the Western United samples of water, soil, sediment, eggs, or animal States—salinity, DDT, and the trace tissue for chemical analysis, they face a elements arsenic, boron, copper, mercury, challenge in determining the sig-nificance of the molybdenum, selenium, and zinc. -

Carpenter Ants and Control in Homes Page 1 of 6
Carpenter Ants and Control in Homes Page 1 of 6 Carpenter Ants and Control in Homes Fact Sheet No. 31 Revised May 2000 Dr. Jay B Karren, Extension Entomologist Alan H. Roe, Insect Diagnostician Introduction Carpenter ants are members of the insect order Hymenoptera, which includes bees, wasps, sawflies, and other ants. Carpenter ants can be occasional pests in the home and are noted particularly for the damage they can cause when nesting in wood. In Utah they are more of a nuisance rather than a major structural pest. Carpenter ants, along with a number of other ant species, utilize cavities in wood, particularly stumps and logs in decayed condition, as nesting sites. They are most abundant in forests and can be easily found under loose bark of dead trees, stumps, or fallen logs. Homeowners may bring them into their homes when they transport infested logs from forests to use as firewood. Description Carpenter ants include species that are among the largest ants found in the United States. They are social insects with a complex and well-defined caste system. The worker ants are sterile females and may occur in different sizes (majors and minors). Members of the reproductive caste (fertile males and females) are usually winged prior to mating. All ants develop from eggs deposited by a fertilized female (queen). The eggs hatch into grub-like larvae (immatures) which are fed and cared for by the workers. When fully grown, the larvae spin a cocoon and enter the pupal stage. The pupal stage is a period of transformation from the larva to adult. -

COMBINED LIST of Particularly Hazardous Substances
COMBINED LIST of Particularly Hazardous Substances revised 2/4/2021 IARC list 1 are Carcinogenic to humans list compiled by Hector Acuna, UCSB IARC list Group 2A Probably carcinogenic to humans IARC list Group 2B Possibly carcinogenic to humans If any of the chemicals listed below are used in your research then complete a Standard Operating Procedure (SOP) for the product as described in the Chemical Hygiene Plan. Prop 65 known to cause cancer or reproductive toxicity Material(s) not on the list does not preclude one from completing an SOP. Other extremely toxic chemicals KNOWN Carcinogens from National Toxicology Program (NTP) or other high hazards will require the development of an SOP. Red= added in 2020 or status change Reasonably Anticipated NTP EPA Haz list COMBINED LIST of Particularly Hazardous Substances CAS Source from where the material is listed. 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide Acutely Toxic Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- Acutely Toxic 1-(2-Chloroethyl)-3-(4-methylcyclohexyl)-1-nitrosourea (Methyl-CCNU) Prop 65 KNOWN Carcinogens NTP 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) IARC list Group 2A Reasonably Anticipated NTP 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) (Lomustine) Prop 65 1-(o-Chlorophenyl)thiourea Acutely Toxic 1,1,1,2-Tetrachloroethane IARC list Group 2B 1,1,2,2-Tetrachloroethane Prop 65 IARC list Group 2B 1,1-Dichloro-2,2-bis(p -chloropheny)ethylene (DDE) Prop 65 1,1-Dichloroethane -

Chemicals Implicated in Colony Collapse Disorder
Chemicals Implicated While research is underway to determine the cause of Colony Collapse Disorder (CCD), pesticides have emerged as one of the prime suspects. Recent bans in Europe attest to the growing concerns surrounding pesticide use and honeybee decline. Neonicotinoids Neonicotinoids are a relatively new class of insecticides that share a common mode of action that affect the central nervous system of insects, resulting in paralysis and death. They include imidacloprid, acetamiprid, clothianidin, dinotefuran, nithiazine, thiacloprid and thiamethoxam. According to the EPA, uncertainties have been identified since their initial registration regarding the potential environmental fate and effects of neonicotinoid pesticides, particularly as they relate to pollinators. Studies conducted in the late 1990s suggest that neonicotinic residues can accumulate in pollen and nectar of treated plants and represent a potential risk to pollinators. There is major concern that neonicotinoid pesticides may play a role in recent pollinator declines. Neonicotinoids can also be persistent in the environment, and when used as seed treatments, translocate to residues in pollen and nectar of treated plants. The potential for these residues to affect bees and other pollinators remain uncertain. Despite these uncertainties, neonicotinoids are beginning to dominate the market place, putting pollinators at risk. The case of the neonicotinoids exemplifies two critical problems with current registration procedures and risk assessment methods for pesticides: the reliance on industry-funded science that contradicts peer-reviewed studies and the insufficiency of current risk assessment procedures to account for sublethal effects of pesticides. • Imidacloprid Used in agriculture as foliar and seed treatments, for indoor and outdoor insect control, home gardening and pet products, imidacloprid is the most popular neonicotinoid, first registered in 1994 under the trade names Merit®, Admire®, Advantage TM. -

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Integrated Pest Management Plan 2021-22
Denair Unified School District INTEGRATED PEST MANAGEMENT PLAN Contacts Denair Unified School District 3460 Lester Rd., Denair, CA Mark Hodges (209) 632-7514 Ext 1215 [email protected] District IPM Coordinator Phone Number e-mail address IPM Statement It is the goal of Denair Unified School District to implement IPM by focusing on long-term prevention or suppression of pests through accurate pest identification, by frequent monitoring for pest presence, by applying appropriate action levels, and by making the habitat less conducive to pests using sanitation and mechanical and physical controls. Pesticides that are effective will be used in a manner that minimizes risks to people, property, and the environment, and only after other options have been shown ineffective. Pest Management Objectives: • Focus on long-term pest prevention using minimal pesticides. • Elimination of significant threats caused by pests to the health and safety of students, staff and the public. • Prevention of loss or damage to structures or property by pests. • Protection of environmental quality inside and outside buildings, in playgrounds and athletic areas, and throughout the Denair Unified School District facilities. IPM Team In addition to the IPM Coordinator, other individuals who are involved in purchasing, making IPM decisions, applying pesticides, and complying with the Healthy Schools Act requirements, include: Name Role Mark Hodges Making IPM Decisions Jerri Pierce Recordkeeping, and Making IPM Decisions Daniel Meza Applying Pesticides, Recordkeeping, -

Impact of Imidacloprid and Horticultural Oil on Nonâ•Fitarget
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 8-2007 Impact of Imidacloprid and Horticultural Oil on Non–target Phytophagous and Transient Canopy Insects Associated with Eastern Hemlock, Tsuga canadensis (L.) Carrieré, in the Southern Appalachians Carla Irene Dilling University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Entomology Commons Recommended Citation Dilling, Carla Irene, "Impact of Imidacloprid and Horticultural Oil on Non–target Phytophagous and Transient Canopy Insects Associated with Eastern Hemlock, Tsuga canadensis (L.) Carrieré, in the Southern Appalachians. " Master's Thesis, University of Tennessee, 2007. https://trace.tennessee.edu/utk_gradthes/120 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Carla Irene Dilling entitled "Impact of Imidacloprid and Horticultural Oil on Non–target Phytophagous and Transient Canopy Insects Associated with Eastern Hemlock, Tsuga canadensis (L.) Carrieré, in the Southern Appalachians." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Entomology and Plant Pathology. Paris L. Lambdin, Major Professor We have read this thesis and recommend its acceptance: Jerome Grant, Nathan Sanders, James Rhea, Nicole Labbé Accepted for the Council: Carolyn R. -

The Impact of the Nation's Most Widely Used Insecticides on Birds
The Impact of the Nation’s Most Widely Used Insecticides on Birds Neonicotinoid Insecticides and Birds The Impact of the Nation’s Most Widely Used Insecticides on Birds American Bird Conservancy, March 2013 Grasshopper Sparrow by Luke Seitz Cover photos: Horned Lark and chicks by Middleton Evans; Corn field, stock.xchng, sxc.hu; Calico Pennant dragonfly by David Cappaert, Michigan State University, Bugwood.org 1 Neonicotinoid Insecticides and Birds American Bird Conservancy would like to thank the Turner Foundation, Wallace Genetic Foundation, Jeff and Connie Woodman, Cornell Douglas Foundation and A.W. Berry Foundation for their ongoing support for American Bird Conservancy’s Pesticides Program. Written by Dr. Pierre Mineau and Cynthia Palmer Designed by Stephanie von Blackwood About the Authors Dr. Pierre Mineau began his long and distinguished scientific career studying the effects of persistent organochlorine compounds, like DDT and PCBs, on fish-eating birds. He then became responsible for the Canadian assessment of new and existing pesticides to determine their adverse impacts on wildlife. In 1994 he transitioned from regulatory reviews to full-time research on the environmental impacts of pesticides, achieving the rank of Senior Research Scientist at Environment Canada. Working with international collaborators and graduate students, he works on assessing globally the environmental footprint of pesticides. He also studies how birds are exposed to pesticides and how bird populations respond to pesticide use and agricultural practices. His work includes defining the ecological values of birds in cropland as well as estimating the incidental take of birds from various other human activities. He has written more than 100 peer-reviewed publications and has authored some 200 presentations.