Morphology of the Foramen Magnum in Young Eastern European Adults
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MR Imaging of the Orbital Apex
J Korean Radiol Soc 2000;4 :26 9-0 6 1 6 MR Imaging of the Orbital Apex: An a to m y and Pat h o l o g y 1 Ho Kyu Lee, M.D., Chang Jin Kim, M.D.2, Hyosook Ahn, M.D.3, Ji Hoon Shin, M.D., Choong Gon Choi, M.D., Dae Chul Suh, M.D. The apex of the orbit is basically formed by the optic canal, the superior orbital fis- su r e , and their contents. Space-occupying lesions in this area can result in clinical d- eficits caused by compression of the optic nerve or extraocular muscles. Even vas c u l a r changes in the cavernous sinus can produce a direct mass effect and affect the orbit ap e x. When pathologic changes in this region is suspected, contrast-enhanced MR imaging with fat saturation is very useful. According to the anatomic regions from which the lesions arise, they can be classi- fied as belonging to one of five groups; lesions of the optic nerve-sheath complex, of the conal and intraconal spaces, of the extraconal space and bony orbit, of the cav- ernous sinus or diffuse. The characteristic MR findings of various orbital lesions will be described in this paper. Index words : Orbit, diseases Orbit, MR The apex of the orbit is a complex region which con- tains many nerves, vessels, soft tissues, and bony struc- Anatomy of the orbital apex tures such as the superior orbital fissure and the optic canal (1-3), and is likely to be involved in various dis- The orbital apex region consists of the optic nerve- eases (3). -

The Morphometric Study of Occurrence and Variations of Foramen Ovale S
Research Article The morphometric study of occurrence and variations of foramen ovale S. Ajrish George*, M. S. Thenmozhi ABSTRACT Background: Foramen vale is one of the important foramina present in the sphenoid bone. Anatomically it is located in the greater wing of the sphenoid bone. The foramen ovale is situated posterolateral to the foramen rotundum and anteromedial to the foramen spinosum. The foramen spinosum is present posterior to the foramen ovale. The carotid canal is present posterior and medial to the foramen spinosum and the foramen rotundum is present anterior to the foramen ovale. The structures which pass through the foramen ovale are the mandibular nerve, emissary vein, accessory middle meningeal artery, and lesser petrosal nerve. The sphenoid bone has a body, a pair of greater wing, pair of lesser wing, pair of lateral pterygoid plate, and a pair of medial pterygoid plate. Aim: The study involves the assessment of any additional features in foramen ovale in dry South Indian skulls. Materials and Methods: This study involves examination of dry adult skulls. First, the foramen ovale is located, and then it is carefully examined for presence of alterations and additional features, and is recorded following computing the data and analyzing it. Results: The maximum length of foramen ovale on the right and left was 10.1 mm, 4.3 mm, respectively. The minimum length of the foramen in right and left was 9.1 mm, 3.2 mm, respectively. The maximum width of foramen ovale on the right and left was 4.8 mm and 2.3 mm, respectively. The minimum width of the foramen in the right and the left side was 5.7 mm and 2.9 mm, respectively. -

Implications to Occipital Headache
The Journal of Neuroscience, March 6, 2019 • 39(10):1867–1880 • 1867 Neurobiology of Disease Non-Trigeminal Nociceptive Innervation of the Posterior Dura: Implications to Occipital Headache X Rodrigo Noseda, Agustin Melo-Carrillo, Rony-Reuven Nir, Andrew M. Strassman, and XRami Burstein Department of Anesthesia, Critical Care and Pain Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts 02115 Current understanding of the origin of occipital headache falls short of distinguishing between cause and effect. Most preclinical studies involving trigeminovascular neurons sample neurons that are responsive to stimulation of dural areas in the anterior 2/3 of the cranium and the periorbital skin. Hypothesizing that occipital headache may involve activation of meningeal nociceptors that innervate the posterior 1⁄3 of the dura, we sought to map the origin and course of meningeal nociceptors that innervate the posterior dura overlying the cerebellum. Using AAV-GFP tracing and single-unit recording techniques in male rats, we found that neurons in C2–C3 DRGs innervate the dura of the posterior fossa; that nearly half originate in DRG neurons containing CGRP and TRPV1; that nerve bundles traverse suboccipital muscles before entering the cranium through bony canals and large foramens; that central neurons receiving nociceptive information from the posterior dura are located in C2–C4 spinal cord and that their cutaneous and muscle receptive fields are found around the ears, occipital skin and neck muscles; and that administration of inflammatory mediators to their dural receptive field, sensitize their responses to stimulation of the posterior dura, peri-occipital skin and neck muscles. These findings lend rationale for the common practice of attempting to alleviate migraine headaches by targeting the greater and lesser occipital nerves with anesthetics. -
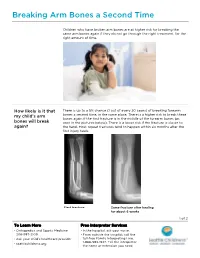
PE2812 Breaking Arm Bones a Second Time
Breaking Arm Bones a Second Time Children who have broken arm bones are at higher risk for breaking the same arm bones again if they do not go through the right treatment, for the right amount of time. How likely is it that There is up to a 5% chance (1 out of every 20 cases) of breaking forearm my child’s arm bones a second time, in the same place. There is a higher risk to break these bones again if the first fracture is in the middle of the forearm bones (as bones will break seen in the pictures below). There is a lower risk if the fracture is closer to again? the hand. Most repeat fractures tend to happen within six months after the first injury heals. First fracture Same fracture after healing for about 6 weeks 1 of 2 To Learn More Free Interpreter Services • Orthopedics and Sports Medicine • In the hospital, ask your nurse. 206-987-2109 • From outside the hospital, call the • Ask your child’s healthcare provider toll-free Family Interpreting Line, 1-866-583-1527. Tell the interpreter • seattlechildrens.org the name or extension you need. Breaking Arm Bones a Second Time How can I help my Wearing a cast for at least six weeks lowers the risk of breaking the same child lower the risk arm bones again. After wearing a cast, we recommend your child wear a brace for 4 weeks in order to protect the injured area and start improving of having a wrist movement. While your child wears a brace, we recommend they do repeated bone not participate in contact sports (e.g., soccer, football or dodge ball). -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -

Biomechanics of Temporo-Parietal Skull Fracture Narayan Yoganandan *, Frank A
Clinical Biomechanics 19 (2004) 225–239 www.elsevier.com/locate/clinbiomech Review Biomechanics of temporo-parietal skull fracture Narayan Yoganandan *, Frank A. Pintar Department of Neurosurgery, Medical College of Wisconsin, 9200 West Wisconsin Avenue, Milwaukee, WI 53226, USA Received 16 December 2003; accepted 16 December 2003 Abstract This paper presents an analysis of research on the biomechanics of head injury with an emphasis on the tolerance of the skull to lateral impacts. The anatomy of this region of the skull is briefly described from a biomechanical perspective. Human cadaver investigations using unembalmed and embalmed and intact and isolated specimens subjected to static and various types of dynamic loading (e.g., drop, impactor) are described. Fracture tolerances in the form of biomechanical variables such as peak force, peak acceleration, and head injury criteria are used in the presentation. Lateral impact data are compared, where possible, with other regions of the cranial vault (e.g., frontal and occipital bones) to provide a perspective on relative variations between different anatomic regions of the human skull. The importance of using appropriate instrumentation to derive injury metrics is underscored to guide future experiments. Relevance A unique advantage of human cadaver tests is the ability to obtain fundamental data for delineating the biomechanics of the structure and establishing tolerance limits. Force–deflection curves and acceleration time histories are used to derive secondary variables such as head injury criteria. These parameters have direct application in safety engineering, for example, in designing vehicular interiors for occupant protection. Differences in regional biomechanical tolerances of the human head have implications in clinical and biomechanical applications. -

GLOSSARY of MEDICAL and ANATOMICAL TERMS
GLOSSARY of MEDICAL and ANATOMICAL TERMS Abbreviations: • A. Arabic • abb. = abbreviation • c. circa = about • F. French • adj. adjective • G. Greek • Ge. German • cf. compare • L. Latin • dim. = diminutive • OF. Old French • ( ) plural form in brackets A-band abb. of anisotropic band G. anisos = unequal + tropos = turning; meaning having not equal properties in every direction; transverse bands in living skeletal muscle which rotate the plane of polarised light, cf. I-band. Abbé, Ernst. 1840-1905. German physicist; mathematical analysis of optics as a basis for constructing better microscopes; devised oil immersion lens; Abbé condenser. absorption L. absorbere = to suck up. acervulus L. = sand, gritty; brain sand (cf. psammoma body). acetylcholine an ester of choline found in many tissue, synapses & neuromuscular junctions, where it is a neural transmitter. acetylcholinesterase enzyme at motor end-plate responsible for rapid destruction of acetylcholine, a neurotransmitter. acidophilic adj. L. acidus = sour + G. philein = to love; affinity for an acidic dye, such as eosin staining cytoplasmic proteins. acinus (-i) L. = a juicy berry, a grape; applied to small, rounded terminal secretory units of compound exocrine glands that have a small lumen (adj. acinar). acrosome G. akron = extremity + soma = body; head of spermatozoon. actin polymer protein filament found in the intracellular cytoskeleton, particularly in the thin (I-) bands of striated muscle. adenohypophysis G. ade = an acorn + hypophyses = an undergrowth; anterior lobe of hypophysis (cf. pituitary). adenoid G. " + -oeides = in form of; in the form of a gland, glandular; the pharyngeal tonsil. adipocyte L. adeps = fat (of an animal) + G. kytos = a container; cells responsible for storage and metabolism of lipids, found in white fat and brown fat. -

98796-Anatomy of the Orbit
Anatomy of the orbit Prof. Pia C Sundgren MD, PhD Department of Diagnostic Radiology, Clinical Sciences, Lund University, Sweden Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lay-out • brief overview of the basic anatomy of the orbit and its structures • the orbit is a complicated structure due to its embryological composition • high number of entities, and diseases due to its composition of ectoderm, surface ectoderm and mesoderm Recommend you to read for more details Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 3 x 3 Imaging technique 3 layers: - neuroectoderm (retina, iris, optic nerve) - surface ectoderm (lens) • CT and / or MR - mesoderm (vascular structures, sclera, choroid) •IOM plane 3 spaces: - pre-septal •thin slices extraconal - post-septal • axial and coronal projections intraconal • CT: soft tissue and bone windows 3 motor nerves: - occulomotor (III) • MR: T1 pre and post, T2, STIR, fat suppression, DWI (?) - trochlear (IV) - abducens (VI) Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Superior orbital fissure • cranial nerves (CN) III, IV, and VI • lacrimal nerve • frontal nerve • nasociliary nerve • orbital branch of middle meningeal artery • recurrent branch of lacrimal artery • superior orbital vein • superior ophthalmic vein Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. -

Required List of Bones and Markings
REQUIRED LIST OF BONES AND MARKINGS Axial Skeleton Skull Cranial Bones (8) Frontal Bone (1) Supraorbital foramina Supraorbital ridges or margins Parietal Bones (2) Temporal Bones (2) External auditory meatus Mastoid process Styloid process Zygomatic process Mandibular fossa Foramen lacerum Carotid foramen Jugular foramen Stylomastoid foramen Internal auditory meatus Occipital Bone (1) Foramen magnum Occipital condyles Ethmoid Bone (1) Cribriform plate Olfactory foramina in cribriform plate Crista galli Perpendicular plate (forms superior part of nasal septum) Middle nasal concha Superior nasal concha Sphenoid Bone (1) Foramen ovale Foramen rotundum Sella turcica Greater wing Lesser wing Optic foramen Inferior orbital fissure Superior orbital fissure Pterygoid processes Skull (cont’d) Facial Bones (14) Lacrimal Bones (2) Lacrimal fossa Nasal Bones (2) Inferior Nasal Conchae (2) Vomer (1) (forms inferior portion of nasal septum) Zygomatic Bones (2) Temporal process (forms zygomatic arch with zygomatic process of temporal bone) Maxillae (2) Alveoli Palatine process (forms anterior part of hard palate) Palatine Bones (2) (form posterior part of hard palate) Mandible (1) Alveoli Body Mental foramen Ramus Condylar process (mandibular condyle) Coronoid process Miscellaneous (Skull) Paranasal sinuses are located in the ethmoid bone, sphenoid bone, frontal bone, and maxillae Zygomatic arch (“cheekbone”) is composed of the zygomatic process of the temporal bone and the temporal process of the zygomatic bone 2 pairs of nasal conchae (superior and middle) are part of the ethmoid bone. 1 pair (inferior) are separate facial bones. All the scroll-like conchae project into the lateral walls of the nasal cavity. Hard palate (“roof of mouth”) is composed of 2 palatine processes of the maxillae and the 2 palatine bones (total of 4 fused bones). -
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled. -

The Skull O Neurocranium, Form and Function O Dermatocranium, Form
Lesson 15 ◊ Lesson Outline: ♦ The Skull o Neurocranium, Form and Function o Dermatocranium, Form and Function o Splanchnocranium, Form and Function • Evolution and Design of Jaws • Fate of the Splanchnocranium ♦ Trends ◊ Objectives: At the end of this lesson, you should be able to: ♦ Describe the structure and function of the neurocranium ♦ Describe the structure and function of the dermatocranium ♦ Describe the origin of the splanchnocranium and discuss the various structures that have evolved from it. ♦ Describe the structure and function of the various structures that have been derived from the splanchnocranium ♦ Discuss various types of jaw suspension and the significance of the differences in each type ◊ References: ♦ Chapter: 9: 162-198 ◊ Reading for Next Lesson: ♦ Chapter: 9: 162-198 The Skull: From an anatomical perspective, the skull is composed of three parts based on the origins of the various components that make up the final product. These are the: Neurocranium (Chondocranium) Dermatocranium Splanchnocranium Each part is distinguished by its ontogenetic and phylogenetic origins although all three work together to produce the skull. The first two are considered part of the Cranial Skeleton. The latter is considered as a separate Visceral Skeleton in our textbook. Many other morphologists include the visceral skeleton as part of the cranial skeleton. This is a complex group of elements that are derived from the ancestral skeleton of the branchial arches and that ultimately gives rise to the jaws and the skeleton of the gill