Implications to Occipital Headache
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Temporo-Mandibular Joint (Tmj) Dysfunction
Office: (310) 423-1220 BeverlyHillsENT.com Fax: (310) 423-1230 TEMPORO-MANDIBULAR JOINT (TMJ) DYSFUNCTION You may not have heard of it, but you use it hundreds of times every day. It is the Temporo- Mandibular Joint (TMJ), the joint where the mandible (the lower jaw) joins the temporal bone of the skull, immediately in front of the ear on each side of your head. You move the joint every time you chew or swallow. You can locate this joint by putting your finger on the triangular structure in front of your ear. Then move your finger just slightly forward and press firmly while you open your jaw all the way and shut it. The motion you feel is the TMJ. You can also feel the joint motion in your ear canal. These maneuvers can cause considerable discomfort to a patient who is having TMJ trouble, and physicians use these maneuvers with patients for diagnosis. TMJ Dysfunction can cause the following symptoms: Ear pain Sore jaw muscles Temple/cheek pain Jaw popping/clicking Locking of the jaw Difficulty in opening the mouth fully Frequent head/neck aches The pain may be sharp and searing, occurring each time you swallow, yawn, talk, or chew, or it may be dull and constant. It hurts over the joint, immediately in front of the ear, but pain can also radiate elsewhere. It often causes spasms in the adjacent muscles that are attached to the bones of the skull, face, and jaws. Then, pain can be felt at the side of the head (the temple), the cheek, the lower jaw, and the teeth. -

Clinical Indicators: Acoustic Neuroma Surgery
Clinical Indicators: Acoustic Neuroma Surgery Approach Procedure CPT Days1 Infratemporal post-auricular approach to middle cranial fossa 61591 90 Transtemporal approach to posterior cranial fossa 61595 90 Transcochlear approach to posterior cranial fossa 61596 90 Transpetrosal approach to posterior cranial fossa 61598 90 Craniectomy for cerebellopontine angle tumor 61520 90 Craniectomy, transtemporal for excision of cerebellopontine angle 61526 90 tumor Combined with middle/posterior fossa craniotomy/ 61530 90 craniectomy Definitive Procedure CPT Days Resection of neoplasm, petrous apex, intradural, including dural 61606 90 repair Resection of neoplasm, posterior cranial fossa, intradural, including 61616 90 repair Microdissection, intracranial 61712 90 Stereotactic radiosurgery 61793 90 Decompression internal auditory canal 69960 90 Removal of tumor, temporal bone middle fossa approach 69970 90 Repair Procedure CPT Days Secondary repair of dura for CSF leak, posterior fossa, by free tissue 61618 90 graft Secondary repair of dura for CSF leak, by local or regional flap or 61619 90 myocutaneous flap Decompression facial nerve, intratemporal; lateral to geniculate 69720 90 ganglion Total facial nerve decompression and/or repair (may include graft) 69955 90 Abdominal fat graft 20926 90 Fascia lata graft; by stripper 20920 90 Fascia lata graft; by incision and area exposure, complex or sheet 20922 90 Intraoperative Nerve Monitoring Procedure CPT Days Auditory nerve monitoring, setup 92585 90 1 RBRVS Global Days Intraoperative neurophysiology testing, hourly 95920 90 Facial nerve monitoring, setup 95925 90 Indications 1. History a) Auditory complaints • hearing loss • fullness • distorted sound perception b) Tinnitus • ringing • humming • hissing • crickets c) Disequilibrium • unsteadiness • dizziness • imbalance • vertigo d) Headache e) Fifth and seventh cranial nerve symptoms • facial pain • facial tingling, numbness • tics • weakness f) Family history of neurofibromatosis type II g) Diplopia h) Dysarthria, dysphasia, aspiration, hoarseness 2. -

Livestock ID Product Catalog Int Ide Cover Ins Not Pr Do Table of Contents
Livestock ID Product Catalog INSIDE COVER DO NOT PRINT Table of Contents Z Tags Z1 No-Snag-Tag® Premium Tags .........................................02 Z Tags The New Z2 No-Tear-Tag™ System .....................................04 We’ve joined forces to bring you Temple Tag Herdsman® Two Piece Tags ........................................06 the best tags in the business. Z Tags Feedlot Tags..........................................................................08 Datamars is a worldwide leader in high-performance livestock identification Temple Tag Feeder Tags ...................................................................10 systems. With its state-of-the-art global manufacturing facilities and Temple Tag Original Tag .................................................................. 12 worldwide technical expertise, Datamars has added Temple Tag® and Z Tags® Temple Tag CalfHerder™ Tag ............................................................ 13 to the company’s global portfolio of world-class brands. Temple Tag FaStocker® Tag ..............................................................14 ComfortEar® Radio Frequency Identification Tags (RFID) ...........16 Datamars can bring you new and improved products faster than ever before. Temple Tag & Z Tags Taggers and Accessories .............................18 But one thing hasn’t changed, and that’s our unwavering commitment to America’s livestock producers. Temple Tag & Z Tags Custom Tag Decoration ..............................20 Temple Tag & Z Tags Hot Stamp Machines ................................... -

Human Sacrifice and Mortuary Treatments in the Great Temple of Tenochtitlán
FAMSI © 2007: Ximena Chávez Balderas Human Sacrifice and Mortuary Treatments in the Great Temple of Tenochtitlán Research Year: 2005 Culture: Mexica Chronology: Postclassic Location: México City, México Site: Tenochtitlán Table of Contents Introduction The bone collection in study Human sacrifice and mortuary treatments Methodology Osteobiography Tafonomic analysis Sacrifice by heart extraction Decapitation: Trophy skulls, Tzompantli skulls and elaboration of skull masks Skull masks Population analysis based upon DNA extraction Preliminary results Final considerations List of Figures Sources Cited Introduction The present report describes the study undertaken with the osteologic collection obtained from the Great Temple excavations in Tenochtitlán; this collection was assembled in the interval from 1978 to 2005. The financial support granted to us by FAMSI enabled the creation of four lines of research: 1) packing and preventive conservation; 2) osteobiography; 3) mortuary treatments; and 4) population genetics. Immediately, a detailed exposition of the work and its results up to the present moment will be given. Submitted 11/16/2006 by: Ximena Chávez Balderas [email protected] Figure 1. General view of the archaeological zone of the Great Temple in México City. 2 The bone collection in study For the present study the human remains found in 19 offerings in the Great Temple of Tenochtitlán were analyzed. This place symbolizes the axis mundi for the Mexicas. The deposits were temporarily situated in the period comprehended between 1440 and 1502 A.D., which corresponds mostly to stage IVb (1469-1481 A.D.). The total number of bodies studied was 1071. From these, seventy-four were recovered in the context of offerings and correspond to skull masks, decapitated skulls, tzompantli skulls, isolated remains and a primary context. -

Knowledge of Skull Base Anatomy and Surgical Implications of Human Sacrifice Among Pre-Columbian Mesoamerican Cultures
See the corresponding retraction, DOI: 10.3171/2018.5.FOCUS12120r, for full details. Neurosurg Focus 33 (2):E1, 2012 Knowledge of skull base anatomy and surgical implications of human sacrifice among pre-Columbian Mesoamerican cultures RAUL LOPEZ-SERNA, M.D.,1 JUAN LUIS GOMEZ-AMADOR, M.D.,1 JUAN BArgES-COLL, M.D.,1 NICASIO ArrIADA-MENDICOA, M.D.,1 SAMUEL ROMERO-VArgAS, M.D., M.SC.,2 MIGUEL RAMOS-PEEK, M.D.,1 MIGUEL ANGEL CELIS-LOPEZ, M.D.,1 ROGELIO REVUELTA-GUTIErrEZ, M.D.,1 AND LESLY PORTOCArrERO-ORTIZ, M.D., M.SC.3 1Department of Neurosurgery, Instituto Nacional de Neurologia y Neurocirugia “Manuel Velasco Suárez;” 2Department of Spine Surgery, Instituto Nacional de Rehabilitación; and 3Department of Neuroendocrinology, Instituto Nacional de Neurologia y Neurocirugia “Manuel Velasco Suárez,” Mexico City, Mexico Human sacrifice became a common cultural trait during the advanced phases of Mesoamerican civilizations. This phenomenon, influenced by complex religious beliefs, included several practices such as decapitation, cranial deformation, and the use of human cranial bones for skull mask manufacturing. Archaeological evidence suggests that all of these practices required specialized knowledge of skull base and upper cervical anatomy. The authors con- ducted a systematic search for information on skull base anatomical and surgical knowledge among Mesoamerican civilizations. A detailed exposition of these results is presented, along with some interesting information extracted from historical documents and pictorial codices to provide a better understanding of skull base surgical practices among these cultures. Paleoforensic evidence from the Great Temple of Tenochtitlan indicates that Aztec priests used a specialized decapitation technique, based on a deep anatomical knowledge. -

Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -

Biomechanics of Temporo-Parietal Skull Fracture Narayan Yoganandan *, Frank A
Clinical Biomechanics 19 (2004) 225–239 www.elsevier.com/locate/clinbiomech Review Biomechanics of temporo-parietal skull fracture Narayan Yoganandan *, Frank A. Pintar Department of Neurosurgery, Medical College of Wisconsin, 9200 West Wisconsin Avenue, Milwaukee, WI 53226, USA Received 16 December 2003; accepted 16 December 2003 Abstract This paper presents an analysis of research on the biomechanics of head injury with an emphasis on the tolerance of the skull to lateral impacts. The anatomy of this region of the skull is briefly described from a biomechanical perspective. Human cadaver investigations using unembalmed and embalmed and intact and isolated specimens subjected to static and various types of dynamic loading (e.g., drop, impactor) are described. Fracture tolerances in the form of biomechanical variables such as peak force, peak acceleration, and head injury criteria are used in the presentation. Lateral impact data are compared, where possible, with other regions of the cranial vault (e.g., frontal and occipital bones) to provide a perspective on relative variations between different anatomic regions of the human skull. The importance of using appropriate instrumentation to derive injury metrics is underscored to guide future experiments. Relevance A unique advantage of human cadaver tests is the ability to obtain fundamental data for delineating the biomechanics of the structure and establishing tolerance limits. Force–deflection curves and acceleration time histories are used to derive secondary variables such as head injury criteria. These parameters have direct application in safety engineering, for example, in designing vehicular interiors for occupant protection. Differences in regional biomechanical tolerances of the human head have implications in clinical and biomechanical applications. -

Morphology of the Foramen Magnum in Young Eastern European Adults
Folia Morphol. Vol. 71, No. 4, pp. 205–216 Copyright © 2012 Via Medica O R I G I N A L A R T I C L E ISSN 0015–5659 www.fm.viamedica.pl Morphology of the foramen magnum in young Eastern European adults F. Burdan1, 2, J. Szumiło3, J. Walocha4, L. Klepacz5, B. Madej1, W. Dworzański1, R. Klepacz3, A. Dworzańska1, E. Czekajska-Chehab6, A. Drop6 1Department of Human Anatomy, Medical University of Lublin, Lublin, Poland 2St. John’s Cancer Centre, Lublin, Poland 3Department of Clinical Pathomorphology, Medical University of Lublin, Lublin, Poland 4Department of Anatomy, Collegium Medicum, Jagiellonian University, Krakow, Poland 5Department of Psychiatry and Behavioural Sciences, Behavioural Health Centre, New York Medical College, Valhalla NY, USA 6Department of General Radiology and Nuclear Medicine, Medical University of Lublin, Lublin, Poland [Received 21 July 2012; Accepted 7 September 2012] Background: The foramen magnum is an important anatomical opening in the base of the skull through which the posterior cranial fossa communicates with the vertebral canal. It is also related to a number of pathological condi- tions including Chiari malformations, various tumours, and occipital dysplasias. The aim of the study was to evaluate the morphology of the foramen magnum in adult individuals in relation to sex. Material and methods: The morphology of the foramen magnum was evalu- ated using 3D computer tomography images in 313 individuals (142 male, 171 female) aged 20–30 years. Results: The mean values of the foramen length (37.06 ± 3.07 vs. 35.47 ± ± 2.60 mm), breadth (32.98 ± 2.78 vs. 30.95 ± 2.71 mm) and area (877.40 ± ± 131.64 vs. -

Surgical Anatomy of the Ligamentous Attachments in the Temple and Periorbital Regions
Cosmetic Surgical Anatomy of the Ligamentous Attachments in the Temple and Periorbital Regions Christopher J. Moss, M.B., B.S., F.R.A.C.S., Dip.Anat., Bryan C. Mendelson, F.R.C.S.(E), F.R.A.C.S., F.A.C.S., and G. Ian Taylor, F.R.C.S., F.R.A.C.S., M.D. Melbourne, Australia This study documents the anatomy of the deep attach- complex system of deep attachments that arise ments of the superficial fasciae within the temporal and from the underlying deep fascia/periosteum. periorbital regions. A highly organized and consistent three-dimensional connective tissue framework supports The subSMAS plane that contains these attach- the overlying skin and soft tissues in these areas. ments is therefore not always a simple cleavage The regional nerves and vessels display constant and plane. This explains why surgical dissection is predictable relationships with both the fascial planes and considerably more complicated in the midfa- their ligamentous attachments. Knowledge of these rela- tionships allows the surgeon to use the tissue planes and cial, temporal, and periorbital regions than in soft-tissue ligaments as intraoperative landmarks for the the scalp. vital neurovascular structures. This results in improved In the cheek, these deep attachments have efficiency and safety for aesthetic procedures in these been defined as the zygomatic, masseteric, and regions. (Plast. Reconstr. Surg. 105: 1475, 2000.) mandibular-cutaneous ligaments.21,22 These lig- aments provide a lateral line of fixation for the mobile tissues of the medial cheek. Release of The patterns of arrangement of the layers of these retaining ligaments is fundamental to the superficial fascia in the cheek,1–9 forehead,10–13 14 15,16 extended SMAS technique of “deep-plane” sur- scalp, and temple have been well de- 18,19,23,24 scribed. -
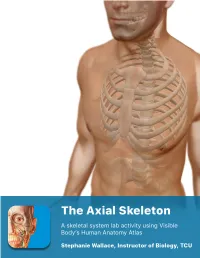
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled. -

Where Is the Temporo-Mandibular Joint?
TMJ Insight into causes and treatments • How does the Temporo-Mandibular Joint work? • What causes TMJ pain? • How is TMJ pain treated? Open your jaw all the way and shut it. This simple movement would not be possible without the Temporo-Mandibular Joint (TMJ). It connects the temporal bone (the bone that forms the side of the skull) and the mandible (the lower jaw). Even though it is only a small disc of cartilage, it separates the bones so that the mandible may slide easily whenever you talk, swallow, chew, kiss, etc. Therefore, damage to this complex, triangular structure in front of your ear, can cause considerable discomfort. Where is the Temporo-Mandibular Joint? You can locate this joint by putting your finger on the triangular structure in front of your ear. Then move your finger just slightly forward and press firmly while you open your jaw all the way and close it. You can also feel the joint motion in your ear canal. How does the TMJ work? When you bite down hard, you put force on the object between your teeth and on the joint. In terms of physics, the jaw is the lever and the TMJ is the fulcrum. Actually, more force is applied (per square foot) to the joint surface than to whatever is between your teeth because the cartilage between the bones provides a smooth surface, over which the joint can freely slide with minimal friction. Therefore, the forces of chewing can be distributed over a wider surface in the joint space and minimize the risk of injury. -
Digital Temple Thermometer KD-2270
Digital Temple Thermometer INSTRUCTIONS FOR USE Please read carefully before using KD-2270 IMPORTANT! BATTERY REPLACEMENT Read instruction manual before using the thermometer Quick Start 1. Take the battery cover off in the direction shown. 1. Install batteries into the thermometer. Make sure the polarity is correct. 2. Install 2 new AAA batteries into thermometer. 2. Press and release the POWER button. Unit will beep once. Wait until it beeps again 3. Re-place the battery cover securely. twice and °F appears in the display. 3. Place and hold the thermometer probe firmly to the skin at the temple and wait Warning : several seconds for the device to beep once more. 1. Do not put used batteries in trash. 4. Read the temperature on the display. 2. Recycle or manage used batteries as hazardous waste. 3. Never dispose of batteries in fire. 4. Dispose of used batteries in recycling trash only. HOW TO USE 5. Do not recharge, put in backwards or disassemble. This may cause explosion, 1. Install 2 AAA batteries. leakage and injury. 2. Press the POWER button to turn on the unit. A beep sound follows. 6. Memory may be erased after replacing a new battery. Power On 3. The last memory is displayed . MEMORY MODE 4. You will hear 2 beeps with the measuring scale shown as following figures. Recalling Memories: Loading Ready to test 1. Turn off the unit first. 2. Press the POWER button and hold for 3 seconds until the last record is shown. 5. Put the thermometer on the temple then it beeps one time to present measurement 3.