Abundance and Diversity of Insects Associated with Citrus Orchards in Two Different Agroecological Zones of Ghana
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

119 Genus Amauris Huebner
AFROTROPICAL BUTTERFLIES 17th edition (2018). MARK C. WILLIAMS. http://www.lepsocafrica.org/?p=publications&s=atb Genus Amauris Hübner, [1816] In: Hübner, [1816-[1826]. Verzeichniss bekannter Schmettlinge 14 (432 + 72 pp.). Augsburg. Type-species: Papilio niavius Linnaeus, by subsequent designation (Scudder, 1875. Proceedings of the American Academy of Arts and Sciences 10: 108 (91-293).). The genus Amauris belongs to the Family Nymphalidae Rafinesque, 1815; Subfamily Danainae Boisduval, 1833; Tribe Danaini Boisduval, 1833; Subtribe Amaurina Le Cerf, 1922. Amauris is the only Afrotropical genus in the Subtribe Amaurina. Amauris is an exclusively Afrotropical genus containing 16 species. Relevant literature: De Vries, 2002 [Differential wing toughness with other taxa]. Amauris species. Final instar larva. Images courtesy Raimund Schutte Amauris species. Pupa. 1 Image courtesy Raimund Schutte Subgenus Amauris Hübner, [1816] In: Hübner, [1816-26]. Verzeichniss bekannter Schmettlinge 14 (432 + 72 pp.). Augsburg. Type-species: Papilio niavius Linnaeus, by subsequent designation (Scudder, 1875. Proceedings of the American Academy of Arts and Sciences 10: 108 (91-293).). *Amauris (Amauris) niavius (Linnaeus, 1758)# Friar Male of the Friar Butterfly (Amauris niavius) at Lake Sibaya, Zululand. Image courtesy Steve Woodhall. Papilio niavius Linnaeus, 1758. Systema Naturae 1, Regnum Animale, 10th edition: 470 (824 pp.). Holmiae. Amauris (Amauris) niavius (Linnaeus, 1758). Pringle et al., 1994: 48. Amauris niavius niavius. Male (Wingspan 75 mm). Left -

Da Guiné-Bissau. Ii. Papilionidae E Pieridae
Boletín Sociedad Entomológica Aragonesa, n1 41 (2007) : 223–236. NOVOS DADOS SOBRE OS LEPIDÓPTEROS DIURNOS (LEPIDOPTERA: HESPERIOIDEA E PAPILIONOIDEA) DA GUINÉ-BISSAU. II. PAPILIONIDAE E PIERIDAE A. Bivar-de-Sousa1, L.F. Mendes2 & S. Consciência3 1 Sociedade Portuguesa de Entomologia, Apartado 8221, 1803-001 Lisboa, Portugal. – [email protected] 2 Instituto de Investigação Científica Tropical (IICT-IP), JBT, Zoologia, R. da Junqueira, 14, 1300-343 Lisboa, Portugal. – [email protected] 3 Instituto de Investigação Científica Tropical (IICT-IP), JBT, Zoologia, R. da Junqueira, 14, 1300-343 Lisboa, Portugal. – [email protected] Resumo: Estudam-se amostras de borboletas diurnas das famílias Papilionidae e Pieridae colhidas ao longo da Guiné-Bissau, no que corresponde à nossa segunda contribuição para o conhecimento das borboletas diurnas deste país. Na sua maioria o material encontra-se depositadas na colecção aracno-entomológica do IICT e na colecção particular do primeiro co-autor, tendo-se reexaminado as amostras determinadas por Bacelar (1949). Em simultâneo, actualizam-se os conhecimentos sobre a fauna de lepidópteros ropalóceros do Parque Natural das Lagoas de Cufada (PNLC). A distribuição geográfica conhecida de cada uma das espécies no país é representada em mapas UTM com quadrícula de 10 Km de lado. Referem-se três espécies de Papilionidae e um género e quatro espécies de Pieridae como novidades faunísticas para a Guiné-Bissau e três espécies de Papilionidae e dois géneros e sete espécies de Pieridae são novas para o PNLC, no total das trinta e uma espécies até ao momento encontradas nestas famílias (nove, e vinte e duas, respectivamente) no país. Palavras chave: Lepidoptera, Papilionidae, Pieridae, distribuição geográfica, Guiné-Bissau. -

Table of Contents
Table of Contents Page LIST OF ACRONYMS a EXECUTIVE SUMMARY I 1.0 Introduction 1 1.1 Scope of Study 1 1.2 Background – Volta River Authority 2 1.3 Proposed Aboadze-Volta Transmission Line Project (AVTP) 3 1.4 Legal, Regulatory and Policy Considerations 5 1.5 Future developments by VRA 8 2.0 Description of proposed development 10 2.1 Pre-Construction Activities 11 2.2 Construction Phase Activities 12 2.3 Operational Phase Activities 17 2.3.1 Other Operational Considerations 20 3.0 Description of Existing Environments 21 3.1 Bio-Physical Environment 21 3.1.1 Climate 21 3.1.2 Flora 25 3.1.3 Fauna 35 3.1.4 Water Resources 43 3.1.5 Geology and Soils 44 3.1.6 General Land Use 51 3.2 Socio-Economic/Cultural Environment 51 3.2.1 Methodology 53 3.2.2 Profiles of the Districts in the Project Area 54 3.2.2(a) Shama - Ahanta East Metropolitan Area 54 3.2.2(b) Komenda - Edina - Eguafo - Abirem (KEEA) District 58 i 3.2.2(c) Mfantseman District 61 3.2.2(d) Awutu-Effutu-Senya District 63 3.2.2(e) Tema Municipal Area 65 3.2.2(f) Abura-Asebu-Kwamankese 68 3.2.2(g) Ga District 71 3.2.2(h) Gomoa District 74 3.3 Results of Socio-Economic Surveys 77 (Communities, Persons and Property) 3.3.1 Information on Affected Persons and Properties 78 3.3.1.1 Age Distribution of Affected Persons 78 3.3.1.2 Gender Distribution of Affected Persons 79 3.3.1.3 Marital Status of Affected Persons 80 3.3.1.4 Ethnic Composition of Afected Persons 81 3.3.1.5 Household Size/Dependents of Affected Persons 81 3.3.1.6 Religious backgrounds of Affected Persons 82 3.3.2 Economic Indicators -

NYMPHALIDAE Nationally As Rare (Range Restricted)
Mecenero et al. / Metamorphosis 31(4): 1–160 134 DOI: https://dx.doi.org/10.4314/met.v31i4.6 localities for this species. This taxon thus qualifies globally under the IUCN criteria as Least Concern and is classified FAMILY: NYMPHALIDAE nationally as Rare (Range Restricted). Genus Cassionympha Dickson, 1981. Change in status from SABCA: The status has not changed from the previous assessment. Cassionympha camdeboo (Dickson, [1981]) Camdeboo Dull Brown; Kamdeboo Bosbruintjie Threats: No threats at present. Ernest L. Pringle Conservation measures and research required: No conservation actions recommended. Research is required LC into its taxonomy, life history and ecology. Better Rare – Restricted Range appreciation of its distribution and subpopulation sizes is Endemic needed. Cassionympha perissinottoi Pringle, 2013 Southern Rainforest Dull Brown; Kusbruintjie Ernest L. Pringle LC Rare – Restricted Range, Habitat Specialist Endemic Type locality: Eastern Cape province: Aberdeen. Taxonomy: There are no notable issues. Distribution: Endemic to the Eastern Cape province of South Africa, in the Aberdeen district. Habitat: Comparatively moist woodland and scrub at high altitude. Vegetation types: NKl2 Eastern Lower Karoo, NKu2 Upper Type locality: Cape Aghulas, Western Cape. Karoo Hardeveld. Taxonomy: Although there is no lack of clarity about the Assessment rationale: This is a range restricted endemic differences between this taxon and its close congeners, all species found in the Eastern Cape province, South Africa 2 records from the southern Cape for Cassionympha cassius (EOO 30 km ). There are two known subpopulations, which and C. detecta will have to be reexamined, because many are not threatened and are in remote areas. Further could represent this new species. -
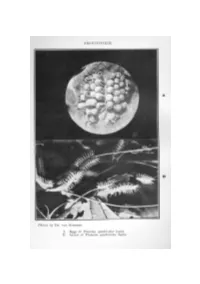
The Butterflies of Kenya and Uganda, Part 5
FRONTISPIECE. A B Photos by DR. VAN SOMEREN. A. Eggs of Planema quadricolor leptis. B. Larval of Planema quadricolor leptis. THE BUTTERFLIES OF KENYA AND UGANDA. By V. G. L. VANSOMEBEN,F.E.S., F.L.S., etc., and REV. K. ST. A. ROGERS,M.A., F.E.S. PARTV. SUB-FAMILYAORiEINiE. GENUSPLANEMA, Dbld. & Hew. INTRODUCTION. Representatives of the genus Planema are to be distinguished from Acuea, by the colour of the Palps, which are black with a lateral grey or white line, and by the position of the first branch of the subcostal or 11th vein; this is given off, at or beyond the apex of the cell. The hind-wing cell is usually much smaller than in ACTma. The ~arvre of the two genera are not markedly different, but those of Planema usually have much longer branched spines and on the whole I1relessparticoloured. The pupre are distinguishable how• ever; those of Planema always have long dorsal spines which are hooked at the end, but the body of the pupa is not heavily ornamented with dark markings. Most of the repre.sentatives of this genus are conspicuously coloured, many having a general superficial resemblance to each other. All are credited with a high degree of distastefulness to enemies, and as a reE\ult quite a number /l.re PUP.IE OF PLANEMA mimicked by species of other genera and families. Some of the most wonderful examples of mimicry are to be found between species of Planema and the Nymphalid8, P8euda()1la3a; exampleE\ are given later. 29 PLANEMA POGGEI NELSON!, Smth. -

Phylogenetic Relationships and Historical Biogeography of Tribes and Genera in the Subfamily Nymphalinae (Lepidoptera: Nymphalidae)
Blackwell Science, LtdOxford, UKBIJBiological Journal of the Linnean Society 0024-4066The Linnean Society of London, 2005? 2005 862 227251 Original Article PHYLOGENY OF NYMPHALINAE N. WAHLBERG ET AL Biological Journal of the Linnean Society, 2005, 86, 227–251. With 5 figures . Phylogenetic relationships and historical biogeography of tribes and genera in the subfamily Nymphalinae (Lepidoptera: Nymphalidae) NIKLAS WAHLBERG1*, ANDREW V. Z. BROWER2 and SÖREN NYLIN1 1Department of Zoology, Stockholm University, S-106 91 Stockholm, Sweden 2Department of Zoology, Oregon State University, Corvallis, Oregon 97331–2907, USA Received 10 January 2004; accepted for publication 12 November 2004 We infer for the first time the phylogenetic relationships of genera and tribes in the ecologically and evolutionarily well-studied subfamily Nymphalinae using DNA sequence data from three genes: 1450 bp of cytochrome oxidase subunit I (COI) (in the mitochondrial genome), 1077 bp of elongation factor 1-alpha (EF1-a) and 400–403 bp of wing- less (both in the nuclear genome). We explore the influence of each gene region on the support given to each node of the most parsimonious tree derived from a combined analysis of all three genes using Partitioned Bremer Support. We also explore the influence of assuming equal weights for all characters in the combined analysis by investigating the stability of clades to different transition/transversion weighting schemes. We find many strongly supported and stable clades in the Nymphalinae. We are also able to identify ‘rogue’ -

Coleoptera: Scarabaeidae)
Systematic Entomology (2005), 31, 113–144 DOI: 10.1111/j.1365-3113.2005.00307.x The phylogeny of Sericini and their position within the Scarabaeidae based on morphological characters (Coleoptera: Scarabaeidae) DIRK AHRENS Deutsches Entomologisches Institut im Zentrum fu¨r Agrarlandschafts- und Landnutzungsforschung Mu¨ncheberg, Germany Abstract. To reconstruct the phylogeny of the Sericini and their systematic position among the scarabaeid beetles, cladistic analyses were performed using 107 morphological characters from the adults and larvae of forty-nine extant scarabaeid genera. Taxa represent most ‘traditional’ subfamilies of coprophagous and phytophagous Scarabaeidae, with emphasis on the Sericini and other melo- lonthine lineages. Several poorly studied exoskeletal features have been examined, including the elytral base, posterior wing venation, mouth parts, endosternites, coxal articulation, and genitalia. The results of the analysis strongly support the monophyly of the ‘orphnine group’ þ ‘melolonthine group’ including phytopha- gous scarabs such as Dynastinae, Hopliinae, Melolonthinae, Rutelinae, and Cetoniinae. This clade was identified as the sister group to the ‘dung beetle line’ represented by Aphodius þ Copris. The ‘melolonthine group’ is comprised in the strict consensus tree by two major clades and two minor lineages, with the included taxa of Euchirinae, Rutelinae, and Dynastinae nested together in one of the major clades (‘melolonthine group I’). Melolonthini, Cetoniinae, and Rutelinae are strongly supported, whereas Melolonthinae and Pachydemini appear to be paraphyletic. Sericini þ Ablaberini were identified to be sister taxa nested within the second major melolonthine clade (‘melolonthine group II’). As this clade is distributed primarily in the southern continents, one could assume that Sericini þ Ablaberini are derived from a southern lineage. -

Contribution to the Lepidopterans of Visakhapatnam Region, Andhra Pradesh, India
ANALYSIS Vol. 21, Issue 68, 2020 ANALYSIS ARTICLE ISSN 2319–5746 EISSN 2319–5754 Species Contribution to the Lepidopterans of Visakhapatnam Region, Andhra Pradesh, India Solomon Raju AJ1, Venkata Ramana K2 1Department of Environmental Sciences, Andhra University, Visakhapatnam 530 003, India 2Department of Botany, Andhra University, Visakhapatnam 530 003, India Corresponding author: A.J. Solomon Raju, Department of Environmental Sciences, Andhra University, Visakhapatnam 530 003, India Mobile: 91-9866256682, email: [email protected] Article History Received: 28 June 2020 Accepted: 02 August 2020 Published: August 2020 Citation Solomon Raju AJ, Venkata Ramana K. Contribution to the Lepidopterans of Visakhapatnam Region, Andhra Pradesh, India. Species, 2020, 21(68), 275-280 Publication License This work is licensed under a Creative Commons Attribution 4.0 International License. General Note Article is recommended to print as color digital version in recycled paper. ABSTRACT The butterflies Byblia ilithyia (Nymphalidae), Pieris canidia (Pieridae) and Azanus jesous (Lycaenidae) and the day-flying moth, Nyctemera adversata (Erebidae) are oligophagous. Previously, only B. ilithyia has been reported to be occurring in this region while the other three species are being reported for the first time from this region. The larval host plants include Jatropha gossypiifolia and Tragia involucrata for B. ilithyia, Brassica oleracea var. oleracea and B. oleracea var. botrytis for P. canidia, and Acacia auriculiformis for Azanus jesous. The nectar plants include Tragia involucrata, Euphorbia hirta and Jatropha gossypiifolia for B. ilithyia, Premna latifolia and Cleome viscosa for P. canidia, Lagascea mollis, Tridax procumbens and Digera muricata for A. jesous and Bidens pilosa for 275 N. adversata. The study recommends extensive field investigations to find out more larval plants and nectar plants for each Page lepidopteran species now reported. -

Check-List of the Butterflies of the Kakamega Forest Nature Reserve in Western Kenya (Lepidoptera: Hesperioidea, Papilionoidea)
Nachr. entomol. Ver. Apollo, N. F. 25 (4): 161–174 (2004) 161 Check-list of the butterflies of the Kakamega Forest Nature Reserve in western Kenya (Lepidoptera: Hesperioidea, Papilionoidea) Lars Kühne, Steve C. Collins and Wanja Kinuthia1 Lars Kühne, Museum für Naturkunde der Humboldt-Universität zu Berlin, Invalidenstraße 43, D-10115 Berlin, Germany; email: [email protected] Steve C. Collins, African Butterfly Research Institute, P.O. Box 14308, Nairobi, Kenya Dr. Wanja Kinuthia, Department of Invertebrate Zoology, National Museums of Kenya, P.O. Box 40658, Nairobi, Kenya Abstract: All species of butterflies recorded from the Kaka- list it was clear that thorough investigation of scientific mega Forest N.R. in western Kenya are listed for the first collections can produce a very sound list of the occur- time. The check-list is based mainly on the collection of ring species in a relatively short time. The information A.B.R.I. (African Butterfly Research Institute, Nairobi). Furthermore records from the collection of the National density is frequently underestimated and collection data Museum of Kenya (Nairobi), the BIOTA-project and from offers a description of species diversity within a local literature were included in this list. In total 491 species or area, in particular with reference to rapid measurement 55 % of approximately 900 Kenyan species could be veri- of biodiversity (Trueman & Cranston 1997, Danks 1998, fied for the area. 31 species were not recorded before from Trojan 2000). Kenyan territory, 9 of them were described as new since the appearance of the book by Larsen (1996). The kind of list being produced here represents an information source for the total species diversity of the Checkliste der Tagfalter des Kakamega-Waldschutzge- Kakamega forest. -

Catalogue of the Type Specimens of Lepidoptera Rhopalocera in the Hill Museum
Original from and digitized by National University of Singapore Libraries Original from and digitized by National University of Singapore Libraries Original from and digitized by National University of Singapore Libraries Original from and digitized by National University of Singapore Libraries CATALOGUE OF THE Type Specimens of Lepidoptera Rhopalocera IN THE HILL MUSEUM BY A. G. GABRIEL, F.E.S. Issued June, 1932 LONDON JOHN BALE, SONS & DANIELSSON, LTD. 83-91, GBEAT TITCHFIELD STEEET, OXEOED STEEET, W. 1 1932 Price 20/- Original from and digitized by National University of Singapore Libraries Unfortunately Mr. Joicey did not live to see the publication of this Catalogue. It will however remain, together with the four completed volumes of the " Bulletin of the Hill Museum," as a lasting memorial to to the magnificent collection of Lepidoptera amassed by Mr. Joicey, and to the work carried out at the Hill Museum under his auspices. G. Talbot. Original from and digitized by National University of Singapore Libraries CATALOGUE OF THE TYPE SPECIMENS OF LEPIDOPTERA RHOPALOCERA IN THE HILL MUSEUM. By A. G. GABRIEL, F.E.S. INTRODUCTION BY G. TALBOT. It is important to know exactly where type specimens are to be found. The British Museum set an example by publishing catalogues of some of their Rhopalocera types, and we hope this will be continued. Mr. Gabriel, who was responsible for that work, has been asked by Mr. Joicey to prepare a catalogue for the Hill Museum. The original description of almost every name in this catalogue has been examined for the correct reference, and where the sex or habitat was wrongly quoted, the necessary correction has been made. -

First Description of White Grub Betle, Maladera Insanabilis Brenske, 1894 (Coleoptera: Melolonthidae: Melolonthinae) from Erbil Governorate, Kurdistan Region – Iraq
Plant Archives Vol. 19 No. 2, 2019 pp. 3991-3994 e-ISSN:2581-6063 (online), ISSN:0972-5210 FIRST DESCRIPTION OF WHITE GRUB BETLE, MALADERA INSANABILIS BRENSKE, 1894 (COLEOPTERA: MELOLONTHIDAE: MELOLONTHINAE) FROM ERBIL GOVERNORATE, KURDISTAN REGION – IRAQ Zayoor Zainel Omar Plant Protection Department, Khabat Technical institute, Erbil Polytechnic University-Erbil, Iraq. Abstract White grub beetle, Maladera insanabilis Brenske, 1894 is collected from the flowers of some ornamental plants in different localities of Erbil governorate, Kurdistan Region-Iraq, from the period, March till July / 2018. Diagnostic characters of the species are figured, Mandibles high sclerotized, irregular shaped, apical part with seven short teeth. apical part of galea with seven well developed teeth. Antenna brown consist of 10 ending in a unilateral three lamellate club sub-equal in length. Fore tibia flattened, bidentate. Parameres is a symmetrical, the left part is hook like, the end with a curved pointed apical tooth. Key words: Maladera insanabilis Brenske, 1894, Kurdistan Region-Iraq. Introduction Woodruff and Beck, 1989, Coca-Abia et al., 1993, Coca- Melolonthidae Samouelle, 1819 is one of large family Abia and Martin-Piera, 1998, Coca-Abia, 2000, Evans, of Scarabaeoidea, there are currently about 750 genera 2003). and 11.000 species recorded worldwide (Houston and The genus Maladera Mulsant and Rey, 1871 is one Weir, 1992 ). The identified of the family is well established of the largest groups consisting of more than 500 and is based on the following characteristics. Adult described species widely distributed in Palearctic, Oriental antennae are lamellate apex, the fore legs are adapted and Afrotropical regions (Ahrens, 2003). -

Phylogeny of Ensifera (Hexapoda: Orthoptera) Using Three Ribosomal Loci, with Implications for the Evolution of Acoustic Communication
Molecular Phylogenetics and Evolution 38 (2006) 510–530 www.elsevier.com/locate/ympev Phylogeny of Ensifera (Hexapoda: Orthoptera) using three ribosomal loci, with implications for the evolution of acoustic communication M.C. Jost a,*, K.L. Shaw b a Department of Organismic and Evolutionary Biology, Harvard University, USA b Department of Biology, University of Maryland, College Park, MD, USA Received 9 May 2005; revised 27 September 2005; accepted 4 October 2005 Available online 16 November 2005 Abstract Representatives of the Orthopteran suborder Ensifera (crickets, katydids, and related insects) are well known for acoustic signals pro- duced in the contexts of courtship and mate recognition. We present a phylogenetic estimate of Ensifera for a sample of 51 taxonomically diverse exemplars, using sequences from 18S, 28S, and 16S rRNA. The results support a monophyletic Ensifera, monophyly of most ensiferan families, and the superfamily Gryllacridoidea which would include Stenopelmatidae, Anostostomatidae, Gryllacrididae, and Lezina. Schizodactylidae was recovered as the sister lineage to Grylloidea, and both Rhaphidophoridae and Tettigoniidae were found to be more closely related to Grylloidea than has been suggested by prior studies. The ambidextrously stridulating haglid Cyphoderris was found to be basal (or sister) to a clade that contains both Grylloidea and Tettigoniidae. Tree comparison tests with the concatenated molecular data found our phylogeny to be significantly better at explaining our data than three recent phylogenetic hypotheses based on morphological characters. A high degree of conflict exists between the molecular and morphological data, possibly indicating that much homoplasy is present in Ensifera, particularly in acoustic structures. In contrast to prior evolutionary hypotheses based on most parsi- monious ancestral state reconstructions, we propose that tegminal stridulation and tibial tympana are ancestral to Ensifera and were lost multiple times, especially within the Gryllidae.