Arise by Chance As the Result of Mutation. They Therefore Suggest
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

119 Genus Amauris Huebner
AFROTROPICAL BUTTERFLIES 17th edition (2018). MARK C. WILLIAMS. http://www.lepsocafrica.org/?p=publications&s=atb Genus Amauris Hübner, [1816] In: Hübner, [1816-[1826]. Verzeichniss bekannter Schmettlinge 14 (432 + 72 pp.). Augsburg. Type-species: Papilio niavius Linnaeus, by subsequent designation (Scudder, 1875. Proceedings of the American Academy of Arts and Sciences 10: 108 (91-293).). The genus Amauris belongs to the Family Nymphalidae Rafinesque, 1815; Subfamily Danainae Boisduval, 1833; Tribe Danaini Boisduval, 1833; Subtribe Amaurina Le Cerf, 1922. Amauris is the only Afrotropical genus in the Subtribe Amaurina. Amauris is an exclusively Afrotropical genus containing 16 species. Relevant literature: De Vries, 2002 [Differential wing toughness with other taxa]. Amauris species. Final instar larva. Images courtesy Raimund Schutte Amauris species. Pupa. 1 Image courtesy Raimund Schutte Subgenus Amauris Hübner, [1816] In: Hübner, [1816-26]. Verzeichniss bekannter Schmettlinge 14 (432 + 72 pp.). Augsburg. Type-species: Papilio niavius Linnaeus, by subsequent designation (Scudder, 1875. Proceedings of the American Academy of Arts and Sciences 10: 108 (91-293).). *Amauris (Amauris) niavius (Linnaeus, 1758)# Friar Male of the Friar Butterfly (Amauris niavius) at Lake Sibaya, Zululand. Image courtesy Steve Woodhall. Papilio niavius Linnaeus, 1758. Systema Naturae 1, Regnum Animale, 10th edition: 470 (824 pp.). Holmiae. Amauris (Amauris) niavius (Linnaeus, 1758). Pringle et al., 1994: 48. Amauris niavius niavius. Male (Wingspan 75 mm). Left -

And Ford, I; Ford, '953) on the Other Hand Have Put Forward a View Intermediate Between the Extreme Ones of Darwin on the One Hand and Goldschmidt on the Other
THE EVOLUTION OF MIMICRY IN THE BUTTERFLY PAPILIO DARDANUS C. A. CLARKE and P. M. SHEPPARD Departments of Medicine and Zoology, University of Liverpool Received23.V.59 1.INTRODUCTION WHENBatesputforward the mimicry hypothesis which bears his name, Darwin (1872), although accepting it, had some difficulty in explaining the evolution of the mimetic resemblance of several distinct species to one distasteful model by a series of small changes, a require- ment of his general theory of evolution. He said "it is necessary to suppose in some cases that ancient members belonging to several distinct groups, before they had diverged to their present extent, accidentally resembled a member of another and protected group in a sufficient degree to afford some slight protection; this having given the basis for the subsequent acquisition of the most perfect resemb- lance ". Punnett (1915) realised that the difficulty is even more acute when one is dealing with a polymorphic species whose forms mimic very distantly related models. Knowing that, in those butterflies which had been investigated genetically, the forms differed by single allelomorphs he concluded that the mimicry did not evolve gradually and did not confer any advantage or disadvantage to the individual. He argued that an allelomorph arises at a single step by mutation and that therefore the mimicry also arises by chance at a single step. Goldschmidt (x) although not denying that mimicry confers some advantage to its possessors also maintained that the resemblance arises fully perfected by a single mutation of a gene distinct from that producing the colour pattern in the model, but producing a similar effect in the mimic. -

Microevolution and the Genetics of Populations Microevolution Refers to Varieties Within a Given Type
Chapter 8: Evolution Lesson 8.3: Microevolution and the Genetics of Populations Microevolution refers to varieties within a given type. Change happens within a group, but the descendant is clearly of the same type as the ancestor. This might better be called variation, or adaptation, but the changes are "horizontal" in effect, not "vertical." Such changes might be accomplished by "natural selection," in which a trait within the present variety is selected as the best for a given set of conditions, or accomplished by "artificial selection," such as when dog breeders produce a new breed of dog. Lesson Objectives ● Distinguish what is microevolution and how it affects changes in populations. ● Define gene pool, and explain how to calculate allele frequencies. ● State the Hardy-Weinberg theorem ● Identify the five forces of evolution. Vocabulary ● adaptive radiation ● gene pool ● migration ● allele frequency ● genetic drift ● mutation ● artificial selection ● Hardy-Weinberg theorem ● natural selection ● directional selection ● macroevolution ● population genetics ● disruptive selection ● microevolution ● stabilizing selection ● gene flow Introduction Darwin knew that heritable variations are needed for evolution to occur. However, he knew nothing about Mendel’s laws of genetics. Mendel’s laws were rediscovered in the early 1900s. Only then could scientists fully understand the process of evolution. Microevolution is how individual traits within a population change over time. In order for a population to change, some things must be assumed to be true. In other words, there must be some sort of process happening that causes microevolution. The five ways alleles within a population change over time are natural selection, migration (gene flow), mating, mutations, or genetic drift. -

Check-List of the Butterflies of the Kakamega Forest Nature Reserve in Western Kenya (Lepidoptera: Hesperioidea, Papilionoidea)
Nachr. entomol. Ver. Apollo, N. F. 25 (4): 161–174 (2004) 161 Check-list of the butterflies of the Kakamega Forest Nature Reserve in western Kenya (Lepidoptera: Hesperioidea, Papilionoidea) Lars Kühne, Steve C. Collins and Wanja Kinuthia1 Lars Kühne, Museum für Naturkunde der Humboldt-Universität zu Berlin, Invalidenstraße 43, D-10115 Berlin, Germany; email: [email protected] Steve C. Collins, African Butterfly Research Institute, P.O. Box 14308, Nairobi, Kenya Dr. Wanja Kinuthia, Department of Invertebrate Zoology, National Museums of Kenya, P.O. Box 40658, Nairobi, Kenya Abstract: All species of butterflies recorded from the Kaka- list it was clear that thorough investigation of scientific mega Forest N.R. in western Kenya are listed for the first collections can produce a very sound list of the occur- time. The check-list is based mainly on the collection of ring species in a relatively short time. The information A.B.R.I. (African Butterfly Research Institute, Nairobi). Furthermore records from the collection of the National density is frequently underestimated and collection data Museum of Kenya (Nairobi), the BIOTA-project and from offers a description of species diversity within a local literature were included in this list. In total 491 species or area, in particular with reference to rapid measurement 55 % of approximately 900 Kenyan species could be veri- of biodiversity (Trueman & Cranston 1997, Danks 1998, fied for the area. 31 species were not recorded before from Trojan 2000). Kenyan territory, 9 of them were described as new since the appearance of the book by Larsen (1996). The kind of list being produced here represents an information source for the total species diversity of the Checkliste der Tagfalter des Kakamega-Waldschutzge- Kakamega forest. -

Archiv Für Naturgeschichte
© Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Lepidoptera für 1903. Bearbeitet von Dr. Robert Lucas in Rixdorf bei Berlin. A. Publikationen (Autoren alphabetisch) mit Referaten. Adkin, Robert. Pyrameis cardui, Plusia gamma and Nemophila noc- tuella. The Entomologist, vol. 36. p. 274—276. Agassiz, G. Etüde sur la coloration des ailes des papillons. Lausanne, H. Vallotton u. Toso. 8 °. 31 p. von Aigner-Abafi, A. (1). Variabilität zweier Lepidopterenarten. Verhandlgn. zool.-bot. Ges. Wien, 53. Bd. p. 162—165. I. Argynnis Paphia L. ; IL Larentia bilineata L. — (2). Protoparce convolvuli. Entom. Zeitschr. Guben. 17. Jahrg. p. 22. — (3). Über Mimikry. Gaea. 39. Jhg. p. 166—170, 233—237. — (4). A mimicryröl. Rov. Lapok, vol. X, p. 28—34, 45—53 — (5). A Mimicry. Allat. Kozl. 1902, p. 117—126. — (6). (Über Mimikry). Allgem. Zeitschr. f. Entom. 7. Bd. (Schluß p. 405—409). Über Falterarten, welche auch gesondert von ihrer Umgebung, in ruhendem Zustande eine eigentümliche, das Auge täuschende Form annehmen (Lasiocampa quercifolia [dürres Blatt], Phalera bucephala [zerbrochenes Ästchen], Calocampa exoleta [Stück morschen Holzes]. — [Stabheuschrecke, Acanthoderus]. Raupen, die Meister der Mimikry sind. Nachahmung anderer Tiere. Die Mimik ist in vielen Fällen zwecklos. — Die wenn auch recht geistreichen Mimikry-Theorien sind doch vielleicht nur ein müßiges Spiel der Phantasie. Aitken u. Comber, E. A list of the butterflies of the Konkau. Journ. Bombay Soc. vol. XV. p. 42—55, Suppl. p. 356. Albisson, J. Notes biologiques pour servir ä l'histoire naturelle du Charaxes jasius. Bull. Soc. Etud. Sc. nat. Nimes. T. 30. p. 77—82. Annandale u. Robinson. Siehe unter S w i n h o e. -

Patterns and Power of Phenotypic Selection in Nature
Articles Patterns and Power of Phenotypic Selection in Nature JOEL G. KINGSOLVER AND DAVID W. PFENNIG Phenotypic selection occurs when individuals with certain characteristics produce more surviving offspring than individuals with other characteristics. Although selection is regarded as the chief engine of evolutionary change, scientists have only recently begun to measure its action in the wild. These studies raise numerous questions: How strong is selection, and do different types of traits experience different patterns of selection? Is selection on traits that affect mating success as strong as selection on traits that affect survival? Does selection tend to favor larger body size, and, if so, what are its consequences? We explore these questions and discuss the pitfalls and future prospects of measuring selection in natural populations. Keywords: adaptive landscape, Cope’s rule, natural selection, rapid evolution, sexual selection henotypic selection occurs when individuals with selection on traits that affect survival stronger than on those Pdifferent characteristics (i.e., different phenotypes) that affect only mating success? In this article, we explore these differ in their survival, fecundity, or mating success. The idea and other questions about the patterns and power of phe- of phenotypic selection traces back to Darwin and Wallace notypic selection in nature. (1858), and selection is widely accepted as the primary cause of adaptive evolution within natural populations.Yet Darwin What is selection, and how does it work? never attempted to measure selection in nature, and in the Selection is the nonrandom differential survival or repro- century following the publication of On the Origin of Species duction of phenotypically different individuals. -

Spatial and Matrix Influences on the Biogeography of Insect Taxa in Forest Fragments in Central Uganda
Spatial and matrix influences on the biogeography of insect taxa in forest fragments in central Uganda Perpetra Akite Dissertation for a cotutelle award of Doctor of Philosophy Degree of Makerere University, Uganda and University of Bergen, Norway Makerere University University of Bergen 2016 Department of Biological Sciences, Makerere University Department of Biology, University of Bergen ii DECLARATION OF ORIGINALITY This is my own work and it has never been submitted for any degree award in any University iii TABLE OF CONTENTS DECLARATION OF ORIGINALITY......................................................................................iii LIST OF CONTENTS...............................................................................................................iv ACKNOWLEDGEMENTS.......................................................................................................vi LIST OF PAPERS....................................................................................................................vii Declaration of authors’ contributions…………………….…...……………...……...viii ABSTRACT...............................................................................................................................x BACKGROUND........................................................................................................................1 Problem statement..........................................................................................................……….2 Objectives........................................................................................................................3 -

Butterfly Species Abundances by Site
Main Bait Line Trap Captures ‐ Species Abundances by Site TOTAL Bobiri Owabi Kajease Bonwire Asantemanso Gyakye Kona Total Specimens 8453 1292 2684 596 746 1059 752 1324 Total Species 116 67 82 37 50 60 47 62 Species List Amauris niavius 20100 0 01 Amauris tartarea 10100 0 00 Andronymus hero 11000 0 00 Anthene locuples 10000 1 00 Anthene rubricinctus 10000 0 10 Ariadne enotrea 42100 0 01 Aterica galene 213 25 96 14 17 35 9 17 Bebearia absolon 66 36 15 2 3 7 0 3 Bebearia barce 40000 4 00 Bebearia cocalia 140540 0 05 Bebearia demetra 11000 0 00 Bebearia lucayensis 52000 0 21 Bebearia mandinga 132512 2 01 Bebearia mardania 47 1 22 0 3 10 0 11 Bebearia oxione 101601 2 00 Bebearia paludicola 80220 3 01 Bebearia phantasina 77000 0 00 Bebearia sophus 270 16 143 9 6 60 11 25 Bebearia tentyris 182 127 19 0 1 1 11 23 Bebearia zonara 44 31 3 0 1 0 1 8 Bicyclus abnormis 667 237 287 0 28 4 0 111 Bicyclus dorothea 68 0 15 21 0 10 7 15 Bicyclus funebris 593 149 147 21 80 48 21 127 Bicyclus madetes 440 25 134 7 71 63 53 87 Bicyclus martius 448 33 70 3 87 47 42 166 Bicyclus procora 67 8 57 0 1 0 1 0 Bicyclus safitza 35 2 12 12 1 3 3 2 Bicyclus sandace 229 2 38 50 4 69 39 27 Bicyclus sangmelinae 40 5 34 0 0 1 0 0 Bicyclus taenias 166 17 47 1 10 30 39 22 Bicyclus vulgaris 504 62 75 87 24 106 84 66 Bicyclus xeneas 41 19 8 0 2 0 0 12 Bicyclus zinebi 154 4 43 6 47 3 3 48 Catuna crithea 20200 0 00 Celaenorrhinus galenus 20 0 1 0 16 1 2 0 Celaenorrhinus meditrina 20000 2 00 Charaxes ameliae 10000 0 01 Charaxes anticlea 41200 1 00 Charaxes bipunctatus 110011 -

Appendix 5 - Species List: Butterflies
Appendix 5 - Species list: Butterflies Butterfly species recorded in the Garden Route National Park. Sources: Butler & Terblanche (1997); Marais (1991). Scientific Name Common Name NYMPHALIDAE Danainae Danaus chrysippus subsp. aegyptius African Monarch Amauris echeria subsp. echeria Chief Acraeinae Acraea horta Garden Acraea Satyrinae Bicyclus safitza subsp. safitza Common Bush Brown Cassionympha cassius Rainforest Brown Dira clytus Cape Autumn Widow Pseudonympha magus Silver-bottom Brown Nymphalinae Junonia hierta subsp. cebrene Yellow Pansy Cynthia cardui Painted Lady Cymothoe alcimeda subsp. alcimeda Battling Glider Charaxinae Charaxes varanes subsp. varanes Pearl Emperor Charaxes Charaxes xiphares subsp. xiphares Forest King Charaxes Charaxes karkloof subsp. trimeni Western Karkloof Charaxes Charaxes karkloof subsp. capensis Eastern Cape Karkloof Charaxes LYCAENIDAE Thestor murrayi Murray's Skolly Capys alphaeus subsp. alphaeus Protea Scarlet Aloeides aranda Aranda Copper Aloeides almeida Almeida Copper Aloeides pallida subsp. (juno?) Tsitsikamma Giant Copper Poecilmitis palmus subsp. margueritae Water Opal Cacyreus palemon subsp. palemon Water bronze Leptotes sp. Common Blue Tarucus thespis Fynbos Blue Lampides boeticus Pea Blue Eicochrypsops messapus subsp. messapus Cupreous Blue PIERIDAE Colias electo subsp. electo African Clouded Yellow Catopsilia florella African Migrant Pinacopteryx eriphia Zebra White Belenois aurota African Caper White Belenois zochalia subsp. zochalia Forest White Belenois creona subsp. severina African Common -

Mt Mabu, Mozambique: Biodiversity and Conservation
Darwin Initiative Award 15/036: Monitoring and Managing Biodiversity Loss in South-East Africa's Montane Ecosystems MT MABU, MOZAMBIQUE: BIODIVERSITY AND CONSERVATION November 2012 Jonathan Timberlake, Julian Bayliss, Françoise Dowsett-Lemaire, Colin Congdon, Bill Branch, Steve Collins, Michael Curran, Robert J. Dowsett, Lincoln Fishpool, Jorge Francisco, Tim Harris, Mirjam Kopp & Camila de Sousa ABRI african butterfly research in Forestry Research Institute of Malawi Biodiversity of Mt Mabu, Mozambique, page 2 Front cover: Main camp in lower forest area on Mt Mabu (JB). Frontispiece: View over Mabu forest to north (TT, top); Hermenegildo Matimele plant collecting (TT, middle L); view of Mt Mabu from abandoned tea estate (JT, middle R); butterflies (Lachnoptera ayresii) mating (JB, bottom L); Atheris mabuensis (JB, bottom R). Photo credits: JB – Julian Bayliss CS ‒ Camila de Sousa JT – Jonathan Timberlake TT – Tom Timberlake TH – Tim Harris Suggested citation: Timberlake, J.R., Bayliss, J., Dowsett-Lemaire, F., Congdon, C., Branch, W.R., Collins, S., Curran, M., Dowsett, R.J., Fishpool, L., Francisco, J., Harris, T., Kopp, M. & de Sousa, C. (2012). Mt Mabu, Mozambique: Biodiversity and Conservation. Report produced under the Darwin Initiative Award 15/036. Royal Botanic Gardens, Kew, London. 94 pp. Biodiversity of Mt Mabu, Mozambique, page 3 LIST OF CONTENTS List of Contents .......................................................................................................................... 3 List of Tables ............................................................................................................................. -
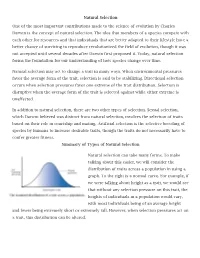
Natural Selection One of the Most Important Contributions Made to The
Natural Selection One of the most important contributions made to the science of evolution by Charles Darwin is the concept of natural selection. The idea that members of a species compete with each other for resources and that individuals that are better adapted to their lifestyle have a better chance of surviving to reproduce revolutionized the field of evolution, though it was not accepted until several decades after Darwin first proposed it. Today, natural selection forms the foundation for our understanding of how species change over time. Natural selection may act to change a trait in many ways. When environmental pressures favor the average form of the trait, selection is said to be stabilizing. Directional selection occurs when selection pressures favor one extreme of the trait distribution. Selection is disruptive when the average form of the trait is selected against while either extreme is unaffected. In addition to natural selection, there are two other types of selection. Sexual selection, which Darwin believed was distinct from natural selection, involves the selection of traits based on their role in courtship and mating. Artificial selection is the selective breeding of species by humans to increase desirable traits, though the traits do not necessarily have to confer greater fitness. Summary of Types of Natural Selection Natural selection can take many forms. To make talking about this easier, we will consider the distribution of traits across a population in using a graph. To the right is a normal curve. For example, if we were talking about height as a trait, we would see that without any selection pressure on this trait, the heights of individuals in a population would vary, with most individuals being of an average height and fewer being extremely short or extremely tall. -

BUTTERFLIES of the CHYULU RANGE. a Systematic List of the Species Taken by the Museum Expedition to the Hills
PART 3. BUTTERFLIES OF THE CHYULU RANGE. A systematic list of the species taken by the Museum Expedition to the Hills. April-July, 1938. By V. G. L. VAN SOMEREN,F.L.S., F.R.E.S., Etc. INTRODUCTION. The following account of the Lepidoptera (Rhopaloc.era) taken by members of the Museum Expedition to the Chyulu Range, is mainly a systematic list of the species obtained. At the time of the visit, April to July, 1938 (that is just toward the end, and after the long rains) insect life was remark• ably scarce, and although systematic search was made over all portions of the hills from 3,000 to 7,000 feet, at no time were butterflies numerous. The material taken can be considered representative of the range for that particular season, but there is little doubt that insect life would be more plentiful just after the short rains, as it undoubtedly is on the surrounding plains, especially in the Kibwezi- Voi areas. In spite of the paucity of insect life, certain new records have been established, thus Papilio hornimani is recorded for the first time from within Kenya boundaries, although known for many years to inhabit the forests of Mt. Kilimanjaro. Charaxes ful• vescens nr. acuminatus, also of Tanganyika, was taken on the range. Two new races of Liptenines of the genus Pentila are recorded, whilst a new ACTaea,a new Papilio, and a new race of Amauris are described. The Lepidoptera collected have a definite relationship to the vegetational zones and the distribution of certain plant species at various altitudes and portions of the hills.