The Role of JAK Inhibitors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CBR-2015.Pdf
MISSION It is the mission of Parkview Medical Center to provide quality healthcare services and education to improve the health of the people we serve. VISION Our vision is to provide healthcare experiences that exceed the expectations of our customers through: • Patient Care • Service • Value The Community Benefit • Clinical Outcomes Report is published by • Education Parkview Medical Center. This information is VALUES intended to inform and Our work values will be governed by Parkview’s core values of: educate about subjects • Integrity and Honesty 400 West 16th Street pertinent to health and • Accountability Pueblo, CO 81003 will be published on an • Continuous Improvement 719.584.4000 annual basis. • Customer Focus • Respect for Individuals • Teamwork proceeds to benefit • Leadership at All Levels • Stewardship COMMUNITY BENEFIT REPORT 3 CARING FOR YOU THE FACTS: Locally-owned & governed Largest employer PATIENTS SERVED: in Pueblo (over 2,600 Serving Pueblo employees) - annual Serving 370,000 County and payroll of $157.9 lives throughout 15,637 Admissions 14 surrounding million Southeastern counties Colorado 136,512 Outpatient visits 4,263 Inpatient surgeries 10,717 Outpatient surgeries 86,922 ER visits , TOTAL THE TEAM: Parkview has a committed team of over 2,600 employees, of which 1,180 have been employed 5 years or more. + + + + + + + + + YEARS YEARS YEARS YEARS YEARS YEARS YEARS YEARS YEARS EMPLOYEES EMPLOYEES EMPLOYEES EMPLOYEES EMPLOYEES EMPLOYEES EMPLOYEES EMPLOYEES EMPLOYEE VOLUNTEERS BOARDS LEADERSHIP NONCLINICAL CLINICAL NURSES MEDICAL 150 volunteers Parkview Boards 63 directors/VPs SUPPORT SUPPORT 769 nurses 372 physicians, worked over included 764 employees 1,100 employees along with 20,000 hours 50 volunteer residents, community fellows, PAs & members NPs Parkview’s incredible journey continues Since it all began in 1923, Parkview Medical Center has provided millions of dollars in charity Community Services care to our community and in fiscal 2015 this Health Education great work continued. -

View a List of the 2021 Performance Achievement Award Recipients
Congratulations to the Recipients of the Rewarding Excellence. Driving Success. The Chest Pain – MI Registry Performance Achievement Award recognizes a hospital’s success in implementing Chest Pain – MI Registry™ a higher standard of care for heart attack patients by meeting aggressive performance measures. 2021 Performance Achievement Award View hospitals participating in the registry at CardioSmart.org/ChestPainMI. St. Francis Medical Center Lee’s Summit Medical Center Summa Health Ascension Seton Medical Center Hays St. Luke’s Hospital Christian Hospital BJC Healthcare JFK Medical Center Baylor Scott & White Heart and Colorado Springs, CO Lee’s Summit, MO Akron, OH Cedar Rapids, IA St. Louis, MO Edison, NJ Vascular – Dallas Kyle, TX Dallas, TX 2021 The George Washington University Hospital SSM Health Saint Louis University Hospital Summa Health 2021 Trinity Medical Center – Bettendorf Citizens Memorial Hospital Ocean Medical Center Washington, DC St. Louis, MO Barberton, OH Dell Seton Medical Center at Bettendorf, IA Bolivar, MO Brick, NJ Baylor Scott & White Medical Center The University of Texas AdventHealth Celebration SSM Health St. Mary’s Hospital – The University of Toledo Medical Center Trinity Regional Medical Center Cox Medical Center Branson Riverview Medical Center – Round Rock Austin, TX Round Rock, TX Kissimmee, FL Jefferson City Toledo, OH Ft. Dodge, IA Branson, MO Red Bank, NJ Jefferson City, MO Houston Methodist The Woodlands Hospital CHRISTUS Mother Frances Hospital Chest Pain – MI Hamilton Medical Center Ascension -

Exhibit #2 Base Rates and Cost-To-Charge Ratios Source: Medicare FY 2019 IPPS Impact File - Correction Notice (September 2018) Effective 1/1/2020
Exhibit #2 Base Rates and Cost-to-Charge Ratios Source: Medicare FY 2019 IPPS Impact File - Correction Notice (September 2018) Effective 1/1/2020 Provider Total Individual Hospital Name Number CCR Base Rate 060001 North Colorado Medical Center 0.268 $6,930.02 060003 Longmont United Hospital 0.323 $6,416.24 060004 Platte Valley Medical Center 0.420 $6,296.29 060006 Montrose Memorial Hospital 0.404 $6,250.18 060008 San Luis Valley Health 0.386 $6,250.18 060009 Lutheran Medical Center 0.235 $6,370.99 060010 Poudre Valley Hospital 0.302 $6,499.23 060011 Denver Health Medical Center 0.324 $8,185.33 060012 Centura Health-St Mary Corwin Medical Center 0.229 $6,818.43 060013 Mercy Regional Medical Center 0.287 $8,029.28 060014 Presbyterian St Lukes Medical Center 0.154 $6,905.48 060015 Centura Health-St Anthony Hospital 0.205 $6,365.32 060020 Parkview Medical Center Inc 0.164 $6,901.98 060022 University Colo Health Memorial Hospital Central 0.221 $6,426.00 060023 St Mary’s Medical Center 0.308 $6,994.17 060024 University Of Colorado Hospital Authority 0.169 $7,902.58 060027 Foothills Hospital 0.218 $6,312.83 060028 Saint Joseph Hospital 0.196 $7,001.47 060030 Mckee Medical Center 0.366 $6,362.47 060031 Centura Health-Penrose St Francis Health Services 0.212 $6,387.05 060032 Rose Medical Center 0.136 $6,735.37 060034 Swedish Medical Center 0.120 $6,539.19 060044 Colorado Plains Medical Center 0.264 $6,621.20 060049 Uchealth Yampa Valley Medical Center 0.539 $9,490.44 060054 Community Hospital 0.322 $6,242.53 060064 Centura Health-Porter Adventist -

2021 Attendees Last Name First Name City State Employer Abbott
2021 Attendees Last Name First Name City State Employer Abbott Jean Aurora CO University of Colorado Anschutz Medical Campus Abbott Patricia Aurora CO University of Colorado Abraham Soney Sunnyvale TX Adams Jill Aurora CO Rocky Mountain Regional VAMC Adams Nicola Colorado Springs CO Dynamic Healthcare Adeniji-Ilesanmi Grace Ooltewah TN Medexpress/Optum Ahlquist-Turner Nola Mayetta KS Prairie Band Potawatomi Nation Health Center Alarcon Rebecca Oakland CA Alfert Susan Oklahoma City OK Allen Julie Birmingham AL UAB Occupational Medicine Anderson Andrea Longmont CO Kaiser Anderson Caleb Ankeny IA Anderson Matthew Taylorsville UT Brigham Young University Anderson-Kather Tiffany Shorewood WI Andrews Terri Zanesville OH Genesis Medical Group Anticuar Samantha Hixson TN Arbuckle Justin H Wheat Ridge CO Children's Eye Physicians Arenson Eileen Lords Valley PA WMH Asch Christina Titusville FL Brevard Medical Dermatology Asher Diane Denver CO Asombrado Doris Carlsbad CA Atkins Dan Aurora CO Children's Hospital Colorado Backer Tammi Littleton CO SCL Health- St Joseph Hospital Bailey Vanessa Frankford WV Balch Nancy Milford MA Massachusetts General Hospital Ballesteros Vynor Las Vegas NV CareMore Medical Group Network Barall Renee Colorado Springs CO Falcon Urgent Care Barch Carol A Mountain View CA Stanford Hospital and Clinics Barrett K.A Kings Park NY Bass Curtis Tolleson AZ Terros Health Bauer Jodi Crested Butte CO Gunnison Valley Health Family Medicine Clinic Beal Geneva Overland Park KS Beard Amanda Longmont CO Beck-Gifford Michael Fort Collins -

ACOI 2019 Congress on Medical Education for Resident Trainers PRELIMINARY AGENDA
ACOI 2019 Congress on Medical Education for Resident Trainers PRELIMINARY AGENDA Thursday, May 9 Saturday, May 11 6:00 PM - 7:30 PM Welcome Reception 7:30 AM – 8:00 AM Continental Breakfast Friday, May 10 8:00 AM - 8:05 AM Opening Comments/Review of the Day 7:30 AM - 5:00 PM Registration Susan M. Enright, DO, FACOI Joanne Kaiser-Smith, DO, FACOI 7:30 AM- 8 AM Continental Breakfast Co-Moderators 8:00 AM – 8:05 AM Welcome & Overview 8:05 AM-8:30 AM Update from the ACOI Council on Susan M. Enright, DO, FACOI Education and Evaluation Joanne Kaiser-Smith, DO, FACOI 2020 AOA Sunsetting Policies Co-Moderators Susan M. Enright, DO, FACOI Joanne Kaiser-Smith, DO, FACOI GENERAL SESSIONS 8:05 AM- 9:15 AM ACGME Update: New Issues/ GENERAL SESSIONS Frequent Citations 8:00 AM – 10:15 AM Program Coordinators Jerry Vasilias, PhD Professional Development • TAGME/Professional 9:15 AM-10:30 AM Priming the Pump for Essential Intern Skills Development Opportunities Philip C. Dittmer, MD • Program Director Calendar – AOA and ACGME 10:30 AM– 10:45 AM Break (Visit Poster Presentations) • Sharing Best Practices • How to Get Involved in Research 10:45 AM- 12:45 PM Utilizing a Flipped Classroom in a • Wellness Opportunities Residency Program • Being Part of the Leadership Team? Andrew King, MD Joanne Kaiser-Smith, DO, FACOI Simiao Li-Sauerwein, MD, MS Moderator Karen Welding-Brown, MPH, CHES 12:45 PM – 1:45 PM Lunch Break (on your own) Kara R. Kerns, ACOI Post-Doctoral Training Specialists 1:45 PM – 2:30 PM How to Respond to ACGME Citations Jill R. -

Hospital Centers of Excellence (Coe)
HOSPITAL CENTERS OF EXCELLENCE (COE) Cigna Health Care – National Centers of Excellence by state and MSA Report 2018–2019 States in blue represent where these COE hospitals WA MT ME are located. ND OR MN VT ID NH SD WI NY MA WY MI CT RI NE IA PA NJ NV OH IN MD IL DE UT CO WV DC CA KS MO VA KY NC TN AZ OK NM AR SC NEW MEXICO GA MS AL LA + TX AK TEX FL HI HI Report as of 01/09/19 and is subject to change. Please see the myCigna.com provider directory for the most current listing and look for the COE icon. Center of Excellence 904780 b 02/19 Metropolitan State Hospital Name Statistical Area Abdominal Hysterectomy Surgery Bariatric Conditions Cancer and Cath Cardiac Angioplasty Surgery Colon Deliveries Laparoscopic Gallbladder Removal Heart Surgery Joint Replacement Orthopedic Back Surgery Pulmonology Medical Total Grand AK Anchorage, AK ALASka NATIVE MEDICAL CENTER X 1 AL Albertville, AL MARSHALL MEDICAL CENTER NORTH X 1 MARSHALL MEDICAL CENTER SOUTH X 1 Alexander City, AL RUSSELL HOSPITAL X X 2 RUSSELL MEDICAL CENTER X X 2 Anniston-Oxford, AL NORTHEAST ALABAMA REGIONAL MEDICAL CENTER X X 2 Auburn-Opelika, AL EAST ALABAMA MEDICAL CENTER X X 2 Birmingham-Hoover, AL GRANDVIEW MEDICAL CENTER X X 2 ST. VINCENt’s BIRMINGHAM X 1 ST. VINCENt’s EAST X X 2 Columbus, GA-AL JACK HUGHSTON MEMORIAL HOSPITAL X 1 Cullman, AL CULLMAN REGIONAL MEDICAL CENTER X 1 Daphne-Fairhope-Foley, AL THOMAS HOSPITAL X X 2 Decatur, AL DECATUR MORGAN HOSPITAL X X X 3 Dothan, AL FLOWERS HOSPITAL X 1 Florence-Muscle Shoals, AL HELEN KELLER HOSPITAL X 1 Mobile, AL MOBILE INFIRMARY MEDICAL CENTER X 1 PROVIDENCE HOSPITAL X X X 3 SPRINGHILL MEMORIAL HOSPITAL X X X 3 Montgomery, AL COMMUNITY HOSPITAL INC. -

Impact of Budget Cuts on Hospital Provider Fee CHA Analysis of Hospital Distribution of $264 Million Cut in SFY 2017-18, As Compared to HPF Payments SFY 2016-17
Impact of Budget Cuts on Hospital Provider Fee CHA analysis of hospital distribution of $264 million cut in SFY 2017-18, as compared to HPF payments SFY 2016-17 HPF CY vs. Hospital HPF PY Animas Surgical Hospital (598,904) Arkansas Valley Regional Medical Center (1,971,032) Aspen Valley Hospital (1,395,504) Banner Health Fort Collins 787,807 Boulder Community Hospital (1,945,134) Centura Health - Avista Adventist Hospital (4,113,161) Centura Health - Castle Rock Adventist Hospital (42,367) Centura Health - Littleton Adventist Hospital (5,698,851) Centura Health - Longmont United Hospital (4,322,012) Centura Health - Mercy Medical Center (1,587,002) Centura Health - Ortho Colorado 75,518 Centura Health - Parker Adventist Hospital (230,602) Centura Health - Penrose -St. Francis Health Services (2,583,949) Centura Health - Porter Adventist Hospital 566,600 Centura Health - Saint Anthony Central Hospital (316,436) Centura Health - Saint Anthony North Hospital (2,277,701) Centura Health - Saint Anthony Summit Hospital (997,859) Centura Health - St. Mary-Corwin Medical Center (2,783,649) Centura Health - St. Thomas More Hospital (3,294,446) Children's Hospital Colorado (17,359,377) Colorado Acute Long Term Hospital (36,532) Colorado Plains Medical Center (2,118,473) Community Hospital (438,146) Conejos County Hospital (970,807) Craig Hospital (336,741) Delta County Memorial Hospital (1,018,926) Denver Health Medical Center (52,575,864) East Morgan County Hospital (1,184,393) Estes Park Medical Center 19,055 Family Health West Hospital (748,787) Good Samaritan Medical Center (771,956) Grand River Medical Center (1,158,011) Gunnison Valley Hospital (464,159) Haxtun Hospital (456,910) HealthOne Medical Center of Aurora 3,899,556 HealthOne North Suburban Medical Center 3,392,897 HealthOne Presbyterian/St. -

Rental Application
RECENT PHOTO (Optional) 400 W. 16th St. Graduate Medical Education Pueblo, CO 81003 (719) 584-4711 (719)595-7972 Fax APPLICATION FOR TRAINING Type of Training Requested: (Check one) _____ Medical Student __________________ from ___________TO ___________ (Specialty) ______ Residency _____________________ from ___________TO ___________ (Specialty) (Please Print or Type) Applicant Information Name: Date of birth: AOA # AAMC# Current Home address: City: State: ZIP Code: Permanent Home Address: City: State: ZIP Code: Telephone Number (Include Area Code) Email Address: Education Pre-Medical Education Name of School: Address: (City and State) Years Attended: (Month and Year) Degree Major: Date: (Month and Year) NameEarned: of School: Address: (City and State) Years Attended: (Month and Year) Degree Major: Date: (Month and Year) MedicalEarned: Education Name of School: Years Attended: (Month and Year) Address: Graduation Date: Degree: (Month and (City and State) (Earned/Expected) Year) Exam Scores COMLEX SCO USMLE ATTEMPS SCHEDULED SCORE ATTEMPTS SCHEDULED SCORES RE SCORES COMLEX USMLE Level 1 Step 1 COMLEX USMLE Level 2 CK Step 2 CK COMLEX USMLE Leve; 2 CS Step 2 CS USMLE Step 3 Page 1 of 4 400 W. 16th St. Graduate Medical Education Pueblo, CO 81003 (719) 584-4711 (719)595-7972 Fax Applicants Name: __________________________________________ Miscellaneous Information Yes No Yes No BCLS Exp. Date: Are you a BCLS or ACLS Certified ACLSAC Exp. Date ArmedInstructor? Service Obligation? Public Health Obligation? Have you ever been convicted of a felony or misdemeanor other than traffic violations? If Yes, please describe: To be completed by Residency or Sub-Specialty Applicants Only: License Information Colorado Physician License # (if you have one) Exp. -

Program Type Totals Pulmonary & Critical Care 170 Sleep 84
Program Type Totals Pulmonary & Critical Care 170 Sleep 84 Pulmonary 22 Critical Care 36 Pediatric Pulmonary 54 Pediatric Critical Care 67 Allergy & Immunology 79 Neonatal-Perinatal 100 Preventive Medicine 76 Pulmonary Disease And Critical Care Medicine Programs Academic Year 2017-2018 United States List of accredited programs within this specialty and those newly accredited programs with future effective dates Program Number / Name Address Program Director Accreditation Effective Core/Related Program Core/Related Status Date Specialty [1563521043] Albany Medical Center Albany Medical Center Department of Medicine MC Scott H. Beegle, MD, Continued 1/19/2018 [1403531248] Albany Medical Center Program Internal medicine Program 17 43 New Scotland Avenue Albany NY, 12208 MS Accreditation Raymond P. Smith, MD [email protected] Ph: (518) 262-5196 Fax: (518) 262-6472 [email protected] [1564113127] Albert Einstein Medical Albert Einstein Medical Center Sunil Sharma, MD Continued 1/19/2018 [1404111369] Albert Einstein Medical Center Internal medicine Center Program 5401 Old York Road, Klein 363 Accreditation Program Philadelphia PA, 19141 Glenn Eiger, MD [email protected] Ph: (215) 456-4555 Fax: (215) 456-1766 EdmondsK@einstein edu [1564121139] Allegheny Health Network Allegheny General Hospital 320 East North Avenue Anil C. Singh, MD Continued 1/19/2018 [1404111381] Allegheny Health Network Medical Internal medicine Medical Education Consortium (AGH) Pittsburgh PA, 15212 Accreditation Education Consortium (AGH) Program Program Ph: (412) 359-5136 James B. Reilly, MD, MS Fax: (412) 442-2139 [email protected] [1560514151] Arrowhead Regional Medical 400 North Pepper Ave Sarkis Arabian, DO Initial Accreditation 7/1/2017 [1400500915] Arrowhead Regional Medical Internal medicine Center Program Colton CA, 92324 Center Program Ph: (909) 580-6343 Debra D. -
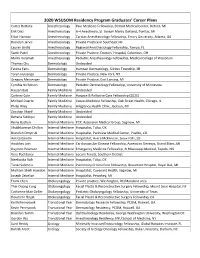
2020 WSUSOM Residency Program Graduates' Career Plans
2020 WSUSOM Residency Program Graduates’ Career Plans Carter Battista Anesthesiology Pain Medicine Fellowship, Detroit Medical Center, Detroit, MI Erik Diaz Anesthesiology A-4 Anesthesia, St. Joseph Mercy Oakland, Pontiac, MI Elliot Harmon Anesthesiology Cardiac Anesthesiology Fellowship, Emory University, Atlanta, GA Brandon Jarvis Anesthesiology Private Practice in Southeast MI Lauren Jindia Anesthesiology Regional Anesthesiology Fellowship, Tampa, FL Samir Patel Anesthesiology Private Practice, Doctors’ Hospital, Columbus, OH Malini Velamati Anesthesiology Pediatric Anesthesiology Fellowship, Medical College of Wisconsin Thomas Chu Dermatology Undecided Fatima Fahs Dermatology Hamzavi Dermatology, Clinton Township, MI Taryn Huizenga Dermatology Private Practice, New York, NY Gregory Messenger Dermatology Private Practice, East Lansing, MI Cynthia Nicholson Dermatology Pediatric Dermatology Fellowship, University of Minnesota Hassan Baiz Family Medicine Undecided Cortney Cole Family Medicine Hospice & Palliative Care Fellowship (2021) Michael Duarte Family Medicine Values Medicine Fellowship, Oak Street Health, Chicago, IL Philip Riley Family Medicine Allegiance Health Clinic, Jackson, MI Zeeshan Sharif Family Medicine Undecided Rehana Siddiqui Family Medicine Undecided Reina Badran Internal Medicine PCP, Ascension Medical Group, Saginaw, MI Shubhkarman Dhillon Internal Medicine Hospitalist, Tulsa, OK Brandon Dmytruk Internal Medicine Hospitalist, Parkview Medical Center, Pueblo, CO Ankita Gandhi Internal Medicine Hospitalist, Avera -

Annals Social Team (AST) Anna Olson, MD, FACEP Change of Shift
Annals Social Team (AST) Anna Olson, MD, FACEP Change of Shift Social Media Parkview Medical Center Pueblo, CO, USA Dr. Olson is Medical Director for Emergency Services and Vice President of Clinical Affairs at Parkview Medical Center in Pueblo, Colorado. She completed her residency in emergency medicine at Denver Health Medical Center in Colorado after attending medical school at Upstate Medical University in Syracuse, New York. She comes to the Social Media arena through literature and the arts, and also edits the Change of Shift column for Annals of Emergency Medicine. Professional interests include process control, improvement, and sustainability, whole-hospital throughput, and departmental efficiency. Seth Trueger, MD, MPH Assistant Social Media Editor University of Chicago @AnnalsofEM tweets signed: "-ST" Podcast and/or Web site: MDaware.org Twitter: @MDaware Seth Trueger, MD, MPH, is a member of the faculty at the University of Chicago. He completed his residency in emergency medicine at the Mount Sinai School of Medicine in New York, and recently completed a Health Policy Fellowship in the Department of Emergency Medicine at George Washington University in Washington, DC. His areas of interest include emergency department crowding and payment reform, airway management, and social media for health professions. Steve Carroll, DO @AnnalsofEM tweets signed: "-SC" Podcast and/or Web site: EM Basic podcast, available on iTunes and at embasic.org Twitter: @embasic Dr. Carroll is an attending physician with the US Army at San Antonio Military Medical Center (SAMMC), where he completed his residency. His areas of interest include emergency medicine education, podcasting, and airway management. His views are his own and do not represent those of the US Army, the Department of Defense, or SAMMC. -

CMS Elections
Inside CMS Be an informed voter, visit: www.cms.org/ CMS elections articles/2017-cms-all-member-election Candidate statement: Henrique Jose Fernandez, MD, FACP, AMA Delegate University of Connecticut, I was elect- in Colorado. In 2016, I was elected by ed to serve as chief medical resident; the entire Parkview Medical Center’s this experience taught me the impor- medical staff to be chief of medicine of tance to lead and teach by example. the institution. After my graduation, I went to the University of Miami, Fla. to pursue my My leadership and vocational time has gastroenterology fellowship. not only been at my institution; since I came to Colorado, I have also been After the completion of my fellow- actively involved at the Pueblo Coun- ship in 2010, Parkview Medical Cen- ty Medical Society, initially as board ter gave me the honor to practice my member and since 2016, I have been career; the hospital has been honor- its president. In my role as president, ing me with different opportunities to I have been working hard to represent grow as physician-gastroenterologist the society at state, regional and na- and in the medical administration- tional levels. leadership field. In the year 2010, I started as board member of the CME Something very important to me is Henrique Jose Fernandez, MD, FACP and GME committees. In the year that throughout my career, my patients Candidate for AMA delegate 2011, I was elected as board member and coworkers have honored me with of the Medical Executive Committee different awards: Fellow of the Ameri- To be a successful academic gastroen- and since then I have been re-elected can College of Physicians, American terologist requires not only the skills for that position.