ACQUIRED HEMOPHILIA Revised Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protein C and S Deficiency in Deep Vein Thrombosis Patients Referred to Iranian Blood Transfusion Organization, Kermanshah
Protein C and S Deficiency in Deep Vein Thrombosis Patients Referred to Iranian Blood Transfusion Organization, Kermanshah Mehrdad Payandeh, 1 Mohammad Erfan Zare, 1, 2 Atefeh Nasir Kansestani, 1, 2 Kamran Ma nsouri, 1, 3 Zohreh Rahimi, 1, 4 Amir Hossein Hashemian, 5 Ebrahim Soltanian, 6 Hoshang Yousefi, 6 1Medical Biology Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran 2Student Research Committee, Kermanshah University of Medical Scien ces, Kermanshah, Iran 3Department of Molecular Medicine, School of advanced Medical Technologies, Tehran University of Medical Sciences, Tehran, Iran 4Department of Biochemistry, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Ir an 5Department of Biostatistics, Faculty of Public Health, Kermanshah University of Medical Sciences, Kermanshah, Iran 6Research Center of Iranian Blood Transfusion Organization, Kermanshah, Iran Corresponding Author : Mohammad Erfan Zare, BSC student of M edical Lab Sciences. Medical Biology Research Center, P.O.Box: 1568, Sorkheh Lizheh, Kermanshah University of Medical Sciences, Kermanshah, Iran. E-mail : [email protected] Tel: +98 831 4276473 Fax: +98 831 4276471 Abstract Introduction: Normal homeostas is system has several inhibitor mechanisms in front of the amplifier’s natural clotting enzyme to prevent fibrin clots in the vessels. The main inhibitors of coagulation pathway are antithrombin (AT), protein C and protein S. Patients with hereditary defic iency of coagulation inhibitors are susceptible to venous thromboembolism (VTE). One of the major clinical manifestations of VTE is deep vein thrombosis (DVT). The present study has investigated the frequency of protein C and S deficiency among DVT patients that by using of these results and results from our previous study; we determined the most important hereditary risk factors for DVT in the Kermanshah Province of Iran with the Kurdish ethnic background. -
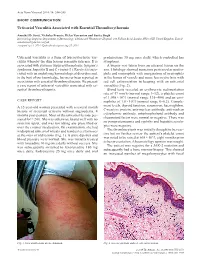
Urticarial Vasculitis Associated with Essential Thrombocythaemia
Acta Derm Venereol 2014; 94: 244–245 SHORT COMMUNICATION Urticarial Vasculitis Associated with Essential Thrombocythaemia Annabel D. Scott, Nicholas Francis, Helen Yarranton and Sarita Singh Dermatology Registrar, Department of Dermatology, Chelsea and Westminster Hospital, 369 Fulham Road, London SW10 9NH, United Kingdom. E-mail: [email protected] Accepted Apr 3, 2013; Epub ahead of print Aug 27, 2013 Urticarial vasculitis is a form of leucocytoclastic vas- prednisolone 30 mg once daily, which controlled her culitis whereby the skin lesions resemble urticaria. It is symptoms. associated with systemic lupus erythematosus, Sjögren’s A biopsy was taken from an uticarial lesion on the syndrome, hepatitis B and C viruses (1). Rarely it is asso- arm. Histology showed numerous perivascular neutro- ciated with an underlying haematological disorders and, phils and eosinophils with margination of neutrophils to the best of our knowledge, has never been reported in in the lumen of vessels and some leucocytoclasis with association with essential thrombocythaemia. We present red cell extravasation in keeping with an urticarial a case report of urticarial vasculitis associated with es- vasculitis (Fig. 2). sential thrombocythaemia. Blood tests revealed an erythrocyte sedimentation rate of 47 mm/h (normal range 1–12), a platelet count of 1,098 × 109/l (normal range 135–400) and an eosi- CASE REPORT nophilia of 1.0 × 109/l (normal range 0–0.2). Comple- A 32-year-old woman presented with a several month ment levels, thyroid function, serum iron, haemoglobin, history of recurrent urticaria without angioedema, 4 C-reactive protein, anti-nuclear antibody, anti-nuclear months post-partum. -

Understanding Haemophilia
Understanding haemophilia Understanding haemophilia Contents Introduction 3 Haemophilia and your child 4 What is haemophilia? 5 What causes haemophilia? 5 Can females have haemophilia? 6 Carriers 8 Who is affected by haemophilia? 9 How severe is haemophilia? 9 Signs and symptoms of haemophilia 11 How is haemophilia diagnosed? 14 Diagnosis 14 Treatment 16 Port-a-cath 19 Managing joint bleeds with PRICE 19 Gene therapy 21 Possible complications of haemophilia 22 Inhibitors 22 Joint damage 22 Medical and dental treatment 23 Surgery Circumcision Dental care Medicines Vaccinations Bleeding disorder card Living with haemophilia 26 Sport and exercise 27 School, college and work 28 Travel 29 Pregnancy and haemophilia 30 Glossary of terms 32 About The Haemophilia Society 33 2 Understanding haemophilia Introduction This booklet is about haemophilia A and B. It gives a general overview of haemophilia and information on diagnosing, treating and living with the condition that we hope will answer your main questions. It has been written for people directly affected by haemophilia and for anyone interested in learning about haemophilia. If you are a parent and your child has recently been diagnosed with haemophilia you may be feeling quite overwhelmed. Remember, you’re not alone and many families are facing the same concerns and issues. Please do get in touch – we have lots of support and information available as well as services for parents and children. You can find out more via our website or Facebook pages, by emailing [email protected] or calling us on 020 7939 0780. The outlook is now the best it has ever been for people with haemophilia in the UK. -

Understanding Haemophilia CHAPTER 2
Understanding haemophilia CHAPTER 2 KEY POINTS • Haemophilia is an inherited condition caused by a gene alteration. • There are two types of haemophilia – A and B. • Haemophilia can be mild, moderate or severe. • Haemophilia is most commonly diagnosed in boys. • If you are considering having more children, there is support available to help with your decision. Haemophilia is an inherited bleeding disorder where blood doesn’t clot properly. It is caused when blood does not produce enough of one of the essential clotting ingredients. These ‘ingredients’ are clotting factors — proteins in the blood that control bleeding. The missing ingredient that causes haemophilia is usually either factor VIII (8) or IX (9). Roman numerals are used when referring to clotting factors. CHAPTER 2 2.1 UNDERSTANDING HAEMOPHILIA Blood clotting and bleeding Understanding how bleeding starts and stops NormalNormal clotting clotting process process Clotting factor activity Source: Hemophilia in Pictures. © WFH 2005. http://www1.wfh.org/publications/files/pdf-1311.pdf Bleeding starts when a capillary (small blood vessel) is injured and blood leaks out. When this happens, the capillary tightens up to slow the bleeding and blood cells called platelets make a plug to patch the hole. For people without haemophilia, the many clotting factors in plasma (part of the blood) knit together to make a clot over the plug. This makes the plug stronger and stops the bleeding. Clotting factor VIII and factor IX are essential to making the blood clot. 2.2 CHAPTER 2 UNDERSTANDING HAEMOPHILIA ClottingClotting in in haemophilia haemophilia Clotting factor activity Source: Hemophilia in Pictures. © WFH 2005. -

Terminology Resource File
Terminology Resource File Version 2 July 2012 1 Terminology Resource File This resource file has been compiled and designed by the Northern Assistant Transfusion Practitioner group which was formed in 2008 and who later identified the need for such a file. This resource file is aimed at Assistant Transfusion Practitioners to help them understand the medical terminology and its relevance which they may encounter in the patient’s medical and nursing notes. The resource file will not include all medical complaints or illnesses but will incorporate those which will need to be considered and appreciated if a blood component was to be administered. The authors have taken great care to ensure that the information contained in this document is accurate and up to date. Authors: Jackie Cawthray Carron Fogg Julia Llewellyn Gillian McAnaney Lorna Panter Marsha Whittam Edited by: Denise Watson Document administrator: Janice Robertson ACKNOWLEDGMENTS We would like to acknowledge the following people for providing their valuable feedback on this first edition: Tony Davies Transfusion Liaison Practitioner Rose Gill Transfusion Practitioner Marie Green Transfusion Practitioner Tina Ivel Transfusion Practitioner Terry Perry Transfusion Specialist Janet Ryan Transfusion Practitioner Dr. Hazel Tinegate Consultant Haematologist Reviewed July 2012 Next review due July 2013 Version 2 July 2012 2 Contents Page no. Abbreviation list 6 Abdominal Aortic Aneurysm (AAA) 7 Acidosis 7 Activated Partial Thromboplastin Time (APTT) 7 Acquired Immune Deficiency Syndrome -

Delivery of Treatment for Haemophilia
WHO/HGN/WFH/ISTH/WG/02.6 ENGLISH ONLY Delivery of Treatment for Haemophilia Report of a Joint WHO/WFH/ISTH Meeting London, United Kingdom, 11 - 13 February 2002 Human Genetics Programme, 2002 Management of Noncommunicable Diseases World Health Organization Human Genetics Programme WHO/HGN/WFH/ISTH/WG/02.6 Management of Noncommunicable Diseases ENGLISH ONLY World Health Organization Delivery of Treatment for Haemophilia Report of a Joint WHO/WFH/ISTH Meeting London, United Kingdom, 11- 13 February 2002 Copyright ã WORLD HEALTH ORGANIZATION, 2002 All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. -

Guidelines for the Management of Haemophilia in Australia
Guidelines for the management of haemophilia in Australia A joint project between Australian Haemophilia Centre Directors’ Organisation, and the National Blood Authority, Australia © Australian Haemophilia Centre Directors’ Organisation, 2016. With the exception of any logos and registered trademarks, and where otherwise noted, all material presented in this document is provided under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Australia (http://creativecommons.org/licenses/by-nc-sa/3.0/au/) licence. You are free to copy, communicate and adapt the work for non-commercial purposes, as long as you attribute the authors and distribute any derivative work (i.e. new work based on this work) only under this licence. If you adapt this work in any way or include it in a collection, and publish, distribute or otherwise disseminate that adaptation or collection to the public, it should be attributed in the following way: This work is based on/includes the Australian Haemophilia Centre Directors’ Organisation’s Guidelines for the management of haemophilia in Australia, which is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Australia licence. Where this work is not modified or changed, it should be attributed in the following way: © Australian Haemophilia Centre Directors’ Organisation, 2016. ISBN: 978-09944061-6-3 (print) ISBN: 978-0-9944061-7-0 (electronic) For more information and to request permission to reproduce material: Australian Haemophilia Centre Directors’ Organisation 7 Dene Avenue Malvern East VIC 3145 Telephone: +61 3 9885 1777 Website: www.ahcdo.org.au Disclaimer This document is a general guide to appropriate practice, to be followed subject to the circumstances, clinician’s judgement and patient’s preferences in each individual case. -

Appendix Search Strategy Treatment of Hemophilia.Pdf
Appendix Search strategies Hemophilia – general aspects PubMed (NLM) September 2009 Von Willebrand disease (TiAb) AND Controlled clinical trial (PT) NOT Purpura, Thrombocytopenic (Me) Angiohemophilia (TiAb) Meta analysis (PT) Blood coagulation disorders (Me) Randomized controlled trial (PT) Hemophilia (TiAb) Systematic (SB) Haemophilia (TiAb) Bleeding disorder (TiAb) Random* (Ti) Bleeding disorders (TiAb) OR Control* (Ti) NOT Medline (SB) ("controlled clinical trial"[Publication Type] OR "meta analysis"[Publication Type] OR "randomized controlled trial"[Publication Type] OR systematic[sb] OR ((random*[Title] OR control*[Title]) NOT Medline[sb])) AND ("von Willebrand Disease"[title/abstract] OR "angiohemophilia"[title/Abstract] OR "Blood Coagulation Disorders"[Mesh terms] OR "hemophilia"[title/abstract] OR "haemophilia"[title/abstract] OR "bleeding disorder"[title/abstract] OR "bleeding disorders"[Title/abstract]) NOT "Purpura, Thrombocytopenic"[MeSH Terms] 211 Hemophilia – general aspects Embase.com (Elsevier) September 2009 Blood clotting factor deficiency (Exp,MJR) AND Clinical trial (Exp) NOT Thrombocytopenic purpura (Exp) Von Willebrand disease (Ti) Intervention study (De) Angiohemophilia (Ti) Longitudinal study (De) Angiohaemophilia (Ti) Prospective study (De) Hemophilia (Ti) Meta analysis (De) Haemophilia (Ti) Systematic review (De) Bleeding disorder (Ti) Random* (Ti) Bleeding disorders (Ti) Control* (Ti) ('blood clotting factor deficiency'/exp/mjOR 'von willebrand disease':ti OR 'angiohemophilia':ti OR 'angiohaemophilia':ti OR 'hemophilia':ti -

Immune Thrombocytopenic Purpura with Subsequent Development of JAK2 V617F-Positive Essential Thrombocythemia: Case Report
ISSN: 2640-7914 DOI: https://dx.doi.org/10.17352/ahcrr CLINICAL GROUP Received: 13 July, 2021 Case Report Accepted: 03 August, 2021 Published: 04 August, 2021 *Corresponding author: Marisabel Hurtado-Castillo, Immune thrombocytopenic PGY-3, Internal Medicine, Department of Medicine at NYU, 536 Ovington Avenue Apartment 3, Brooklyn NYC 11209, USA, Tel: 718-312-9641; purpura with subsequent Email: Keywords: Immune thrombocytopenic purpura; development of JAK2 Essential thrombocythemia; JAK-STAT signaling path- way; JAK2(V617F) mutation V617F-positive essential https://www.peertechzpublications.com thrombocythemia: Case Report Marisabel Hurtado-Castillo1*, Brian Flaherty2 and Morris Jrada2 1PGY-3, Internal Medicine, Department of Medicine at NYU, Brooklyn, NY, USA 2Clinical Assistant Professor, Department of Medicine at NYU Grossman School of Medicine, Brooklyn, NY, USA Abstract The sequential occurrence of Immune Thrombocytopenic Purpura (ITP) and Essential Thrombocythemia (ET) has been reported in the literature on a few occasions, as these are two hematologic disorders with distinct etiologies and patients usually have contrasting clinical presentations. Our case highlights the sequential occurrence of ITP, followed by Janus kinase 2 (JAK2) (V617F)-positive ET in a 64-year-old white woman, after four years of follow-up. The pathophysiology relating to these two conditions is incompletely understood, however, JAK2(V617F) mutation has been found in all the cases reported. Early identifi cation of JAK2(V617F) mutation in a patient with a -

Thrombocytopenia.Pdf
THROMBOCYTOPENIA DIFFERENTIAL DIAGNOSIS FALSELY LOW PLATELET COUNT In vitro platelet clumping caused by EDTA-dependent agglutinins Giant platelets COMMON CAUSES OF THROMBOCYTOPENIA Pregnancy (gestational thrombocytopenia, preeclampsia) Drug-induced thrombocytopenia (i.e., heparin, quinidine, quinine, and sulfonamides) Viral infections (ie. HIV, rubella, infectious mononucleosis) Hypersplenism due to chronic liver disease Dilutional (massive transfusion) OTHER CAUSES OF THROMBOCYTOPENIA Myelodysplasia Congenital thrombocytopenia Thrombotic thrombocytopenic purpura (TTP) -hemolytic-uremic syndrome (HUS) Chronic disseminated intravascular coagulation (DIC) Autoimmune diseases, such as systemic lupus erythematosus-associated lymphoproliferative disorders (CLL and NHL) Sepsis Idiopathic thrombocytopenic purpura (ITP)* DIFFERENTIAL FOR THROMBOCYTOPENIA BASED ON CLINICAL SETTING CLINICAL SETTING DIFFERENTIAL DIAGNOSES Cardiac surgery Cardiopulmonary bypass, HIT, dilutional thrombocytopenia, PTP Interventional cardiac Abciximab or other IIb/IIIa blockers, HIT procedure Sepsis syndrome DIC, ehrlichiosis, sepsis, hemophagocytosis syndrome, drug-induced, misdiagnosed TTP, mechanical ventilation, pulmonary artery catheters Pulmonary failure DIC, hantavirus pulmonary syndrome, mechanical ventilation, pulmonary artery catheters Mental status TTP, ehrlichiosis changes/seizures Renal failure TTP, Dengue, HIT, DIC, HUS Continuous hemofiltration HIT, consumption by filter and tubing Cardiac failure HIT, drug-induced, pulmonary artery catheter Post-surgery -

Acute Immune Thrombocytopenic Purpura in Children
Turk J Hematol 2007; 24:41-51 REVIEW ARTICLE © Turkish Society of Hematology Acute immune thrombocytopenic purpura in children Abdul Rehman Sadiq Public School, Bahawalpur, Pakistan [email protected] Received: Sep 12, 2006 • Accepted: Mar 21, 2007 ABSTRACT Immune thrombocytopenic purpura (ITP) in children is usually a benign and self-limiting disorder. It may follow a viral infection or immunization and is caused by an inappropriate response of the immune system. The diagnosis relies on the exclusion of other causes of thrombocytopenia. This paper discusses the differential diagnoses and investigations, especially the importance of bone marrow aspiration. The course of the disease and incidence of intracranial hemorrhage are also discussed. There is substantial discrepancy between published guidelines and between clinicians who like to over-treat. The treatment of the disease ranges from observation to drugs like intrave- nous immunoglobulin, steroids and anti-D to splenectomy. The different modes of treatment are evaluated. The best treatment seems to be observation except in severe cases. Key Words: Thrombocytopenic purpura, bone marrow aspiration, Intravenous immunoglobulin therapy, steroids, anti-D immunoglobulins 41 Rehman A INTRODUCTION There is evidence that enhanced T-helper cell/ Immune thrombocytopenic purpura (ITP) in APC interactions in patients with ITP may play an children is usually a self-limiting disorder. The integral role in IgG antiplatelet autoantibody pro- American Society of Hematology (ASH) in 1996 duction -

Painless Purple Streaks on the Arms and Chest
PHOTO CHALLENGE Painless Purple Streaks on the Arms and Chest Tiffany Alexander, MD; Bernard Cohen, MD A 10-year-old boy presented with painless purple streaks on the arms and chest of 2 months’ duration. The rash recurred several times per month and cleared without treatment in 3 to 5 days. There was no history of trauma or medication exposure, and he was growing and developing normally.copy WHAT’S THE DIAGNOSIS? a. child maltreatment syndrome b. notfactitial purpura c. Henoch-Schönlein purpura d. idiopathic thrombocytopenic purpura Doe. meningococcemia PLEASE TURN TO PAGE E9 FOR THE DIAGNOSIS CUTIS Dr. Alexander was from the University of Maryland, Baltimore, and currently is from the Department of Dermatology, Duke University Medical Center, Durham, North Carolina. Dr. Cohen is from the Department of Dermatology, Division of Pediatric Dermatology, Johns Hopkins University School of Medicine, Baltimore. The authors report no conflict of interest. Correspondence: Bernard Cohen, MD, Johns Hopkins University School of Medicine, Division of Pediatric Dermatology, David M. Rubenstein Child Health Bldg, Ste 2107, 200 N Wolfe St, Baltimore, MD 21287 ([email protected]). E8 I CUTIS® WWW.MDEDGE.COM/DERMATOLOGY Copyright Cutis 2019. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. PHOTO CHALLENGE DISCUSSION THE DIAGNOSIS: Factitial Purpura actitial dermatologic disorders are characterized by the state of one’s health and life span.4 Cupping is per- skin findings triggered by deliberate manipulation formed by placing a glass cup over a painful body part. F of the skin with objects to create lesions and feign A partial vacuum is created by flaming, mechanical with- signs of a dermatologic condition to seek emotional drawal, or thermal cooling of the entrapped air under the and psychological benefit.1 The etiology of the lesions is cup.