Iom-Hepatitisandlivercancerreport.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prevention & Control of Viral Hepatitis Infection
Prevention & Control of Viral Hepatitis Infection: A Strategy for Global Action © World Health Organization 2011. All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by WHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. Table of contents Disease burden 02 What is viral hepatitis? 05 Prevention & control: a tailored approach 06 Global Achievements 08 Remaining challenges 10 World Health Assembly: a mandate for comprehensive prevention & control 13 WHO goals and strategy -

The Role of Hepatitis C Virus in Hepatocellular Carcinoma U
Viruses in cancer cell plasticity: the role of hepatitis C virus in hepatocellular carcinoma U. Hibner, D. Gregoire To cite this version: U. Hibner, D. Gregoire. Viruses in cancer cell plasticity: the role of hepatitis C virus in hepato- cellular carcinoma. Contemporary Oncology, Termedia Publishing House, 2015, 19 (1A), pp.A62–7. 10.5114/wo.2014.47132. hal-02187396 HAL Id: hal-02187396 https://hal.archives-ouvertes.fr/hal-02187396 Submitted on 2 Jun 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - ShareAlike| 4.0 International License Review Viruses are considered as causative agents of a significant proportion of human cancers. While the very Viruses in cancer cell plasticity: stringent criteria used for their clas- sification probably lead to an under- estimation, only six human viruses the role of hepatitis C virus are currently classified as oncogenic. In this review we give a brief histor- in hepatocellular carcinoma ical account of the discovery of on- cogenic viruses and then analyse the mechanisms underlying the infectious causes of cancer. We discuss viral strategies that evolved to ensure vi- Urszula Hibner1,2,3, Damien Grégoire1,2,3 rus propagation and spread can alter cellular homeostasis in a way that increases the probability of oncogen- 1Institut de Génétique Moléculaire de Montpellier, CNRS, UMR 5535, Montpellier, France ic transformation and acquisition of 2Université Montpellier 2, Montpellier, France stem cell phenotype. -

Rational Engineering of HCV Vaccines for Humoral Immunity
viruses Review To Include or Occlude: Rational Engineering of HCV Vaccines for Humoral Immunity Felicia Schlotthauer 1,2,†, Joey McGregor 1,2,† and Heidi E Drummer 1,2,3,* 1 Viral Entry and Vaccines Group, Burnet Institute, Melbourne, VIC 3004, Australia; [email protected] (F.S.); [email protected] (J.M.) 2 Department of Microbiology and Immunology, Peter Doherty Institute for Infection and Immunity, University of Melbourne, Melbourne, VIC 3000, Australia 3 Department of Microbiology, Monash University, Clayton, VIC 3800, Australia * Correspondence: [email protected]; Tel.: +61-392-822-179 † These authors contributed equally to this work. Abstract: Direct-acting antiviral agents have proven highly effective at treating existing hepatitis C infections but despite their availability most countries will not reach the World Health Organization targets for elimination of HCV by 2030. A prophylactic vaccine remains a high priority. Whilst early vaccines focused largely on generating T cell immunity, attention is now aimed at vaccines that gen- erate humoral immunity, either alone or in combination with T cell-based vaccines. High-resolution structures of hepatitis C viral glycoproteins and their interaction with monoclonal antibodies isolated from both cleared and chronically infected people, together with advances in vaccine technologies, provide new avenues for vaccine development. Keywords: glycoprotein E2; vaccine development; humoral immunity Citation: Schlotthauer, F.; McGregor, J.; Drummer, H.E To Include or 1. Introduction Occlude: Rational Engineering of HCV Vaccines for Humoral Immunity. The development of direct-acting antiviral agents (DAA) with their ability to cure Viruses 2021, 13, 805. https:// infection in >95% of those treated was heralded as the key to eliminating hepatitis C globally. -
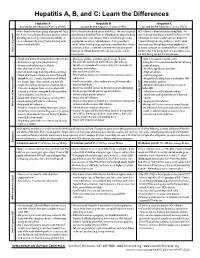
Hepatitis A, B, and C: Learn the Differences
Hepatitis A, B, and C: Learn the Differences Hepatitis A Hepatitis B Hepatitis C caused by the hepatitis A virus (HAV) caused by the hepatitis B virus (HBV) caused by the hepatitis C virus (HCV) HAV is found in the feces (poop) of people with hepa- HBV is found in blood and certain body fluids. The virus is spread HCV is found in blood and certain body fluids. The titis A and is usually spread by close personal contact when blood or body fluid from an infected person enters the body virus is spread when blood or body fluid from an HCV- (including sex or living in the same household). It of a person who is not immune. HBV is spread through having infected person enters another person’s body. HCV can also be spread by eating food or drinking water unprotected sex with an infected person, sharing needles or is spread through sharing needles or “works” when contaminated with HAV. “works” when shooting drugs, exposure to needlesticks or sharps shooting drugs, through exposure to needlesticks on the job, or from an infected mother to her baby during birth. or sharps on the job, or sometimes from an infected How is it spread? Exposure to infected blood in ANY situation can be a risk for mother to her baby during birth. It is possible to trans- transmission. mit HCV during sex, but it is not common. • People who wish to be protected from HAV infection • All infants, children, and teens ages 0 through 18 years There is no vaccine to prevent HCV. -

Should Your Company's Health Care Premiu Cover the Cost of Paper Worl
THE UNIVERSITY OF MINNESOTA Should your company's health care premiu cover the cost of paper worl<? Blue Cross thinks so ... Yes, Blu e Cross t hinks so . And it's no minor co nveni · (Incidentally, last year Minnesota Blue Cross proc· ence . The fact t hat Blue Cross does handle all the paper essed over 200,000 hospital claims.) work of your health care program saves you time and This efficiency of administration does not mean costly personnel . .. this translates into money. you 're getting less for your health care dollar. In fa ct. First, when Blue Cross handles all the paper work, year after year, Blue Cross returns over 90 cents of it saves the expense of office space, files, supplies and every health care dollar to members in the form of expensive man hours. (This cuts your overhead and benefits. frees personnel to handle other important tasks.) Shouldn't you expect your hea lth care premium to Second, when Blue Cross processes claims, you include the cost of paper work? Blue Cross thinks so . have a staff of professionals working for you . They audit every bil l . an efficient check and balance on Why not ca ll the man from Blue Cross? He's an the money you're spending for a health care program . expert in designing health care programs. NOW AVAILABLE A booklet titled "How to Evaluate Group Hospitaliza· tion Today" will be sent to you-no cost or obligation. The booklet wi ll prove helpful to you in ana lyzi ng mii you'r present hospitalization program. -

Understanding Human Astrovirus from Pathogenesis to Treatment
University of Tennessee Health Science Center UTHSC Digital Commons Theses and Dissertations (ETD) College of Graduate Health Sciences 6-2020 Understanding Human Astrovirus from Pathogenesis to Treatment Virginia Hargest University of Tennessee Health Science Center Follow this and additional works at: https://dc.uthsc.edu/dissertations Part of the Diseases Commons, Medical Sciences Commons, and the Viruses Commons Recommended Citation Hargest, Virginia (0000-0003-3883-1232), "Understanding Human Astrovirus from Pathogenesis to Treatment" (2020). Theses and Dissertations (ETD). Paper 523. http://dx.doi.org/10.21007/ etd.cghs.2020.0507. This Dissertation is brought to you for free and open access by the College of Graduate Health Sciences at UTHSC Digital Commons. It has been accepted for inclusion in Theses and Dissertations (ETD) by an authorized administrator of UTHSC Digital Commons. For more information, please contact [email protected]. Understanding Human Astrovirus from Pathogenesis to Treatment Abstract While human astroviruses (HAstV) were discovered nearly 45 years ago, these small positive-sense RNA viruses remain critically understudied. These studies provide fundamental new research on astrovirus pathogenesis and disruption of the gut epithelium by induction of epithelial-mesenchymal transition (EMT) following astrovirus infection. Here we characterize HAstV-induced EMT as an upregulation of SNAI1 and VIM with a down regulation of CDH1 and OCLN, loss of cell-cell junctions most notably at 18 hours post-infection (hpi), and loss of cellular polarity by 24 hpi. While active transforming growth factor- (TGF-) increases during HAstV infection, inhibition of TGF- signaling does not hinder EMT induction. However, HAstV-induced EMT does require active viral replication. -

The Drawings of Cornelis Visscher (1628/9-1658) John Charleton
The Drawings of Cornelis Visscher (1628/9-1658) John Charleton Hawley III Jamaica Plain, MA M.A., History of Art, Institute of Fine Arts – New York University, 2010 B.A., Art History and History, College of William and Mary, 2008 A Dissertation presented to the Graduate Faculty of the University of Virginia in Candidacy for the Degree of Doctor of Philosophy Department of Art and Architectural History University of Virginia May, 2015 _______________________________________ _______________________________________ _______________________________________ _______________________________________ Table of Contents Abstract ............................................................................................................................................. i Acknowledgements.......................................................................................................................... ii Introduction ..................................................................................................................................... 1 Chapter 1: The Life of Cornelis Visscher .......................................................................................... 3 Early Life and Family .................................................................................................................... 4 Artistic Training and Guild Membership ...................................................................................... 9 Move to Amsterdam ................................................................................................................. -

Hepatitis B: Get Tested. Get Vaccinated Booklet
HEPATITIS B GET TESTED. GET VACCINATED. Table of Contents Hepatitis B (Hep B) and Your Liver I 1 Get Tested: Know Your Hep B Status I 5 Get Vaccinated: Protect Yourself and Others I 8 Get Care and Treatment I 8 Living with Hep B I 10 Pregnancy, Children and Hep B I 14 Information and Resources I 16 Hepatitis B (Hep B) and Your Liver Hepatitis means inflammation of the liver. Your liver keeps you healthy in many ways. It removes toxins from your body and transforms nutrients from food into energy. Hep B can cause serious health problems, including liver scarring, liver failure, liver cancer and early death. There are different types of hepatitis. Hep B is caused by the Hep B virus that infects and attacks the liver. Hep B can be prevented with a safe vaccine and can be treated to slow or stop development of serious liver damage. People living with Hep B can live a long and healthy life. 241,000 people in New York City (NYC) are living with Hep B. 2.2 million people in the United States are living with Hep B. 1 How does someone get Hep B? Hep B can be passed from one person to another through blood, semen and vaginal fluids. Some common ways to pass Hep B include: • During childbirth; a pregnant person living with Hep B can pass Hep B to their newborn. • Having sex without a physical barrier, such as condoms or dental dams, with a person living with Hep B. • Sharing or reusing needles, medical or injection equipment, such as for insulin, glucose monitors, drug use, steroids, tattooing or acupuncture. -

Surnames in Bureau of Catholic Indian
RAYNOR MEMORIAL LIBRARIES Montana (MT): Boxes 13-19 (4,928 entries from 11 of 11 schools) New Mexico (NM): Boxes 19-22 (1,603 entries from 6 of 8 schools) North Dakota (ND): Boxes 22-23 (521 entries from 4 of 4 schools) Oklahoma (OK): Boxes 23-26 (3,061 entries from 19 of 20 schools) Oregon (OR): Box 26 (90 entries from 2 of - schools) South Dakota (SD): Boxes 26-29 (2,917 entries from Bureau of Catholic Indian Missions Records 4 of 4 schools) Series 2-1 School Records Washington (WA): Boxes 30-31 (1,251 entries from 5 of - schools) SURNAME MASTER INDEX Wisconsin (WI): Boxes 31-37 (2,365 entries from 8 Over 25,000 surname entries from the BCIM series 2-1 school of 8 schools) attendance records in 15 states, 1890s-1970s Wyoming (WY): Boxes 37-38 (361 entries from 1 of Last updated April 1, 2015 1 school) INTRODUCTION|A|B|C|D|E|F|G|H|I|J|K|L|M|N|O|P|Q|R|S|T|U| Tribes/ Ethnic Groups V|W|X|Y|Z Library of Congress subject headings supplemented by terms from Ethnologue (an online global language database) plus “Unidentified” and “Non-Native.” INTRODUCTION This alphabetized list of surnames includes all Achomawi (5 entries); used for = Pitt River; related spelling vartiations, the tribes/ethnicities noted, the states broad term also used = California where the schools were located, and box numbers of the Acoma (16 entries); related broad term also used = original records. Each entry provides a distinct surname Pueblo variation with one associated tribe/ethnicity, state, and box Apache (464 entries) number, which is repeated as needed for surname Arapaho (281 entries); used for = Arapahoe combinations with multiple spelling variations, ethnic Arikara (18 entries) associations and/or box numbers. -

Making Hepatitis B History 2014 Annual Report
Making Hepatitis B History 2014 Annual Report BARUCH S. BLUMBERG INSTITUTE Hepatitis B Foundation 2014 Annual Report 1 Making Hepatitis B History December 2014 The next three years will be the “make-it” period for the Hepatitis B Foundation and its research arm, the Baruch S. Blumberg Institute. We have imposed an ambitious timeline for results because we believe the opportunity is now, and the need is urgent. Our world-class research institute—renamed in 2013 as the Baruch S. Blumberg Institute in honor of our co-founder and Nobel Laureate Dr. Blumberg—is poised to significantly accelerate the pace of research. We have recruited a group of first class scientists to join us fulltime at the Blumberg Institute in 2015. Our new scientists, however, aren’t starting from zero. Indeed, they will have a head start since we have been preparing and building toward this goal for more than two decades. And all of our scientists have pledged to identify breakthrough therapies for hepatitis B in the next three years. We want, and plan for, our discoveries to go from the lab into human use as quickly as possible. We know that this will require drug development experts with pharmaceutical company experience and we have already made arrangements to create technology development partnerships. This means that our scientists can focus on pioneering discoveries and our partners can carry them all the way to the clinic. Our hope for the next 5 to 10 years is simple—that a complete cure is found, which will benefit everyone living with chronic hepatitis B worldwide. -

Needs of Individuals Living with Hepatitis Delta Virus and Their Caregivers, 2016-2019
Thomas Jefferson University Jefferson Digital Commons Department of Medicine Faculty Papers Department of Medicine 12-17-2020 Needs of Individuals Living With Hepatitis Delta Virus and Their Caregivers, 2016-2019. Priyanka Kumar Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, Pennsylvania; Jefferson College of Population Health, Philadelphia, Pennsylvania Catherine Freeland Jefferson College of Population Health, Philadelphia, Pennsylvania; Hepatitis B Foundation, Doylestown, Pennsylvania Sierra Bodor Hepatitis B Foundation, Doylestown, Pennsylvania Sean Farrell Jefferson College of Population Health, Philadelphia, Pennsylvania; Geisinger Commonwealth School of Medicine, Scranton, Pennsylvania Follow this and additional works at: https://jdc.jefferson.edu/medfp Chari Par Cohent of the Other Medical Specialties Commons LetHepatitis us B Fknowoundation, how Doylest accessown, Pennsylv taniao this document benefits ouy RecommendedSee next page for Citation additional authors Kumar, Priyanka; Freeland, Catherine; Bodor, Sierra; Farrell, Sean; Cohen, Chari; and Frasso, Rosemary PhD, MSc, CPH, "Needs of Individuals Living With Hepatitis Delta Virus and Their Caregivers, 2016-2019." (2020). Department of Medicine Faculty Papers. Paper 284. https://jdc.jefferson.edu/medfp/284 This Article is brought to you for free and open access by the Jefferson Digital Commons. The Jefferson Digital Commons is a service of Thomas Jefferson University's Center for Teaching and Learning (CTL). The Commons is a showcase for Jefferson books and journals, peer-reviewed scholarly publications, unique historical collections from the University archives, and teaching tools. The Jefferson Digital Commons allows researchers and interested readers anywhere in the world to learn about and keep up to date with Jefferson scholarship. This article has been accepted for inclusion in Department of Medicine Faculty Papers by an authorized administrator of the Jefferson Digital Commons. -

Annual Report 2010
2010 ANNUAL REPORT RESEARCH OUTREACH ADVOCACY 3 Hepat itis B Foundation “We appreciate [the HBF’s] continued support of the Hep B community. With the addition of a second adopted child DearThe Hepatitis Friends, B Foundation has enjoyed one of its most with hep B into our family, we obviously 2010 Annual Report productive years despite the economic downturn. We are are supportive of your efforts in pleased to report that our work has been a bright spot in RESEARCH research and education.” one of the toughest years. YEAR IN REVIEW (New Jersey) This year we have focused our efforts on making the FINANCIAL INFORMatioN* greatest impact on some of the greatest unmet needs. (Fiscal Year 2010 ending June 30, 2010) IN THE LABS This includes establishing consensus recommendations Sources of Funds – HBF & IHVR Combined for hepatitis B screening and treatment of children who (Total $4,322,068) live with this disease and for primary care providers who Charitable lack clear guidelines. Grants 84% Contributions 11% $3,649,257 $475,492 Research results take longer, but we have impressive Interest >1% LIVING SCIENCE and tangible results to share, too. This year, the liver $3,157 Building for a Cure cancer biomarker that we helped to discover has been Other 4% $194,162 HBF’s groundbreaking for the expansion optioned to a major diagnostics company. If all goes well, of its research facility to build nine new this blood test will be available for human use within the labs, which includes a teaching lab, • Biomarkers of liver cancer enter next couple of years.