The Role of Hepatitis C Virus in Hepatocellular Carcinoma U
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prevention & Control of Viral Hepatitis Infection
Prevention & Control of Viral Hepatitis Infection: A Strategy for Global Action © World Health Organization 2011. All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by WHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. Table of contents Disease burden 02 What is viral hepatitis? 05 Prevention & control: a tailored approach 06 Global Achievements 08 Remaining challenges 10 World Health Assembly: a mandate for comprehensive prevention & control 13 WHO goals and strategy -

Rational Engineering of HCV Vaccines for Humoral Immunity
viruses Review To Include or Occlude: Rational Engineering of HCV Vaccines for Humoral Immunity Felicia Schlotthauer 1,2,†, Joey McGregor 1,2,† and Heidi E Drummer 1,2,3,* 1 Viral Entry and Vaccines Group, Burnet Institute, Melbourne, VIC 3004, Australia; [email protected] (F.S.); [email protected] (J.M.) 2 Department of Microbiology and Immunology, Peter Doherty Institute for Infection and Immunity, University of Melbourne, Melbourne, VIC 3000, Australia 3 Department of Microbiology, Monash University, Clayton, VIC 3800, Australia * Correspondence: [email protected]; Tel.: +61-392-822-179 † These authors contributed equally to this work. Abstract: Direct-acting antiviral agents have proven highly effective at treating existing hepatitis C infections but despite their availability most countries will not reach the World Health Organization targets for elimination of HCV by 2030. A prophylactic vaccine remains a high priority. Whilst early vaccines focused largely on generating T cell immunity, attention is now aimed at vaccines that gen- erate humoral immunity, either alone or in combination with T cell-based vaccines. High-resolution structures of hepatitis C viral glycoproteins and their interaction with monoclonal antibodies isolated from both cleared and chronically infected people, together with advances in vaccine technologies, provide new avenues for vaccine development. Keywords: glycoprotein E2; vaccine development; humoral immunity Citation: Schlotthauer, F.; McGregor, J.; Drummer, H.E To Include or 1. Introduction Occlude: Rational Engineering of HCV Vaccines for Humoral Immunity. The development of direct-acting antiviral agents (DAA) with their ability to cure Viruses 2021, 13, 805. https:// infection in >95% of those treated was heralded as the key to eliminating hepatitis C globally. -

Hepatitis A, B, and C: Learn the Differences
Hepatitis A, B, and C: Learn the Differences Hepatitis A Hepatitis B Hepatitis C caused by the hepatitis A virus (HAV) caused by the hepatitis B virus (HBV) caused by the hepatitis C virus (HCV) HAV is found in the feces (poop) of people with hepa- HBV is found in blood and certain body fluids. The virus is spread HCV is found in blood and certain body fluids. The titis A and is usually spread by close personal contact when blood or body fluid from an infected person enters the body virus is spread when blood or body fluid from an HCV- (including sex or living in the same household). It of a person who is not immune. HBV is spread through having infected person enters another person’s body. HCV can also be spread by eating food or drinking water unprotected sex with an infected person, sharing needles or is spread through sharing needles or “works” when contaminated with HAV. “works” when shooting drugs, exposure to needlesticks or sharps shooting drugs, through exposure to needlesticks on the job, or from an infected mother to her baby during birth. or sharps on the job, or sometimes from an infected How is it spread? Exposure to infected blood in ANY situation can be a risk for mother to her baby during birth. It is possible to trans- transmission. mit HCV during sex, but it is not common. • People who wish to be protected from HAV infection • All infants, children, and teens ages 0 through 18 years There is no vaccine to prevent HCV. -

Understanding Human Astrovirus from Pathogenesis to Treatment
University of Tennessee Health Science Center UTHSC Digital Commons Theses and Dissertations (ETD) College of Graduate Health Sciences 6-2020 Understanding Human Astrovirus from Pathogenesis to Treatment Virginia Hargest University of Tennessee Health Science Center Follow this and additional works at: https://dc.uthsc.edu/dissertations Part of the Diseases Commons, Medical Sciences Commons, and the Viruses Commons Recommended Citation Hargest, Virginia (0000-0003-3883-1232), "Understanding Human Astrovirus from Pathogenesis to Treatment" (2020). Theses and Dissertations (ETD). Paper 523. http://dx.doi.org/10.21007/ etd.cghs.2020.0507. This Dissertation is brought to you for free and open access by the College of Graduate Health Sciences at UTHSC Digital Commons. It has been accepted for inclusion in Theses and Dissertations (ETD) by an authorized administrator of UTHSC Digital Commons. For more information, please contact [email protected]. Understanding Human Astrovirus from Pathogenesis to Treatment Abstract While human astroviruses (HAstV) were discovered nearly 45 years ago, these small positive-sense RNA viruses remain critically understudied. These studies provide fundamental new research on astrovirus pathogenesis and disruption of the gut epithelium by induction of epithelial-mesenchymal transition (EMT) following astrovirus infection. Here we characterize HAstV-induced EMT as an upregulation of SNAI1 and VIM with a down regulation of CDH1 and OCLN, loss of cell-cell junctions most notably at 18 hours post-infection (hpi), and loss of cellular polarity by 24 hpi. While active transforming growth factor- (TGF-) increases during HAstV infection, inhibition of TGF- signaling does not hinder EMT induction. However, HAstV-induced EMT does require active viral replication. -

Sensitive Detection of Oncoviruses Integrated Into a Comprehensive Tumor Immuno-Genomics Platform #3788
Sensitive detection of oncoviruses integrated into a comprehensive tumor immuno-genomics platform #3788 Gábor Bartha, Robin Li, Shujun Luo, John West, Richard Chen Personalis, Inc. | 1330 O’Brien Dr., Menlo Park, CA 94025 Contact: [email protected] Introduction Results Mixed Oncoviral Cell Lines We obtained 22 cell lines from ATCC containing HPV16, HPV18, HPV45, HPV68, HBV, EBV, KSHV, HTLV1 and HTLV2 in which the oncoviruses HPV, HBV, HCV and EBV viruses are causally EBV Cell Lines were known to be in the tumors from which the cell lines were created. In the ATCC samples we detected 23 out of 23 oncoviruses expected in linked to over 11% of cancers worldwide while both the DNA and RNA. We detected all the different types of oncoviruses that we targeted except for HCV because it wasn’t in any sample. In all KSHV, HTLV and MCV are linked to an additional To test the ability of the platform to detect oncoviruses, we identified a set of 11 EBV cell lines from but one case the signals were strong. 1%. As use of immunotherapy expands to a Coriell in which EBV was used as a transformant. We detected EBV in all the Coriell cell lines in both broader variety of cancers, it is important to DNA and RNA indicating strong sensitivity of the platform. Wide dynamic ranGe suggests quantification Detected in DNA Detected in RNA Virus Tissue Notes understand how these oncoviruses may be may be possible as well. In the DNA and RNA there were no detections of any other oncovirus EBV EBV EBV HodGkin’s lymphoma Per ATCC : “The cells are EBNA positive" indicating high specificity. -

Host Cell Functions in Vesicular Stomatitis Virus Replication Phat X
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Veterinary and Biomedical Science Veterinary and Biomedical Sciences, Department of 2016 Host Cell Functions In Vesicular Stomatitis Virus Replication Phat X. Dinh University of Nebraska - Lincoln Anshuman Das University of Nebraska-Lincoln Asit K. Pattnaik University of Nebraska - Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/vetscipapers Part of the Biochemistry, Biophysics, and Structural Biology Commons, Cell and Developmental Biology Commons, Immunology and Infectious Disease Commons, Medical Sciences Commons, Veterinary Microbiology and Immunobiology Commons, and the Veterinary Pathology and Pathobiology Commons Dinh, Phat X.; Das, Anshuman; and Pattnaik, Asit K., "Host Cell Functions In Vesicular Stomatitis Virus Replication" (2016). Papers in Veterinary and Biomedical Science. 237. https://digitalcommons.unl.edu/vetscipapers/237 This Article is brought to you for free and open access by the Veterinary and Biomedical Sciences, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Papers in Veterinary and Biomedical Science by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Published in Biology and Pathogenesis of Rhabdo and Filoviruses Copyright © 2016 World Scientific Publishing Co Pte Ltd digitalcommons.unl.edu HOST CELL FUNCTIONS IN VESICULAR STOMATITIS VIRUS REPLICATION Phat X. Dinh, Anshuman Das, and Asit K. Pattnaik School of Veterinary Medicine and Biomedical Sciences and the Nebraska Center for Virology, University of Nebraska-Lincoln, 109 Morrison Life Science Research Center, 4240 Fair Street, Lincoln, * Nebraska 68583, USA E-mail: [email protected] Summary Vesicular stomatitis virus (VSV), the prototypic rhabdovirus, has been used as an excellent paradigm for understanding the mechanisms of virus replication, pathogenesis, host response to virus infection and also for myriads of studies on cellular and molecular biology. -

Nucleotide Sequences in Mouse DNA and RNA Specific for Moloney Sarcoma Virus
Proc. Nati. Acad. Sci. USA Vol. 73, No. 10, pp. 3705-3709, October 1976 Microbiology Nucleotide sequences in mouse DNA and RNA specific for Moloney sarcoma virus (murine sarcoma virus/RNA tumor virus/nucleic acid hybridization/gene evolution/sarcoma-specific nucleotide sequence) ARTHUR E. FRANKEL AND PETER J. FISCHINGER Laboratory of Viral Carcinogenesis, National Cancer Institute, Bethesda, Maryland 20014 Communicated by Howard M. Temin, July 12,1976 ABSTRACT Complementary DNA (cDNA) synthesized (3). Laboratory strains of oncoviruses do contain information by Moloney murine sarcoma virus (M-MSV) was separated into that is different from the spontaneously released oncovirus of two parts, the first, termed MSV-specific cDNA, composed of the species Of nucleotide sequences found only in M-MSV viral RNA, and the (6, 7). these, the sarcomagenic oncoviruses gen- second, termed MSV-MuLV common cDNA, composed of nu- erally contain both a set of nucleotide sequences shared with cleotide sequences that were found in both M-MSV and murine leukosis-leukemia viruses, and another set of sequences that are leukemia virus (MuLV) viral RNAs. RNA complementary to the dissimilar from those of other oncoviruses of the species (6-10). MSV-specific cDNA was not found in several other MSV iso- Complementary DNA (cDNA) can be transcribed from sar- lates, nor in ecotropic MuLV, mouse mammary tumor virus, or coma virus RNA by the viral endogenous DNA polymerase. several murine xenotropic oncoviruses. Cellular DNA of several The cDNA represents both the "shared" and the species was examined for the presence of nucleotide sequences "specific" complementary to MSV-specific cDNA. Cells transformed by moieties of the sarcoma virus genome. -

The Role of Hepatitis C Virus in Hepatocellular Carcinoma A63
Review Viruses are considered as causative agents of a significant proportion of human cancers. While the very Viruses in cancer cell plasticity: stringent criteria used for their clas- sification probably lead to an under- estimation, only six human viruses the role of hepatitis C virus are currently classified as oncogenic. In this review we give a brief histor- in hepatocellular carcinoma ical account of the discovery of on- cogenic viruses and then analyse the mechanisms underlying the infectious causes of cancer. We discuss viral strategies that evolved to ensure vi- Urszula Hibner1,2,3, Damien Grégoire1,2,3 rus propagation and spread can alter cellular homeostasis in a way that increases the probability of oncogen- 1Institut de Génétique Moléculaire de Montpellier, CNRS, UMR 5535, Montpellier, France ic transformation and acquisition of 2Université Montpellier 2, Montpellier, France stem cell phenotype. We argue that 3Université Montpellier 1, Montpellier, France a useful way of analysing the conver- gent characteristics of viral infection and cancer is to examine how viruses affect the so-called cancer hallmarks. Introduction This view of infectious origin of cancer It is estimated that close to 20% of human cancers are due to infections is illustrated by examples from hep- atitis C infection, which is associated with known pathogens, mainly with viruses [1]. Given the difficulties in un- with a high proportion of hepatocellu- ambiguously assigning their causative role [2] and the fast rate of discovery lar carcinoma. of new viruses [3], it is likely a conservative estimate. Different pathogens are associated with increased cancer risk. One of the Key words: viruses, cancer cell plas- first identified cancer-causing infectious disease was schistosomiasis, ini- ticity, HCV, hepatocellular carcinoma. -

Revisiting the Elusive Hepatitis C Vaccine
Editorial Revisiting the Elusive Hepatitis C Vaccine Stephen M. Todryk 1,2,* , Margaret F. Bassendine 2,* and Simon H. Bridge 1,2,* 1 Faculty of Health & Life Sciences, Northumbria University, Newcastle upon Tyne NE1 8ST, UK 2 Translational & Clinical Research Institute, The Medical School, Newcastle University, Newcastle upon Tyne NE2 4HH, UK * Correspondence: [email protected] (S.M.T.); [email protected] (M.F.B.); [email protected] (S.H.B.) The impactful discovery and subsequent characterisation of hepatitis C virus (HCV), an RNA virus of the flavivirus family, led to the awarding of the 2020 Nobel Prize in Physiology or Medicine to Harvey J. Alter, Michael Houghton and Charles M. Rice [1]. However, despite the significant advances recognised by this Nobel prize, an effective HCV vaccine remains elusive. The recent success of vaccines against SARS-CoV-2, developed with unprecedented speed, has shone a bright light on the vaccination process for protection against viral threats and may provide renewed impetus for the development of vaccines for other viruses. HCV infection remains a major global health problem as approximately three quarters of those infected develop chronic infection, leading to morbidity and mortality due to progressive liver fibrosis, cirrhosis and cancer. The World Health Organization (WHO) estimates that 71.1 million people are living with chronic HCV, resulting in over 400,000 deaths every year. In 2016, the WHO adopted a global strategy with the aim of eliminating viral hepatitis as a public health problem, comprising targets to reduce new viral hepatitis infections by 90% and reduce deaths due to viral hepatitis by 65% by 2030. -

Cancer Patients Have a Higher Risk Regarding COVID-19–And Vice Versa?
pharmaceuticals Opinion Cancer Patients Have a Higher Risk Regarding COVID-19–and Vice Versa? Franz Geisslinger, Angelika M. Vollmar and Karin Bartel * Pharmaceutical Biology, Department Pharmacy, Ludwig-Maximilians-University of Munich, 81377 Munich, Germany; [email protected] (F.G.); [email protected] (A.M.V.) * Correspondence: [email protected] Received: 29 May 2020; Accepted: 3 July 2020; Published: 6 July 2020 Abstract: The world is currently suffering from a pandemic which has claimed the lives of over 230,000 people to date. The responsible virus is called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and causes the coronavirus disease 2019 (COVID-19), which is mainly characterized by fever, cough and shortness of breath. In severe cases, the disease can lead to respiratory distress syndrome and septic shock, which are mostly fatal for the patient. The severity of disease progression was hypothesized to be related to an overshooting immune response and was correlated with age and comorbidities, including cancer. A lot of research has lately been focused on the pathogenesis and acute consequences of COVID-19. However, the possibility of long-term consequences caused by viral infections which has been shown for other viruses are not to be neglected. In this regard, this opinion discusses the interplay of SARS-CoV-2 infection and cancer with special focus on the inflammatory immune response and tissue damage caused by infection. We summarize the available literature on COVID-19 suggesting an increased risk for severe disease progression in cancer patients, and we discuss the possibility that SARS-CoV-2 could contribute to cancer development. -

Human Papillomaviruses and Epstein–Barr Virus Interactions in Colorectal Cancer: a Brief Review
pathogens Review Human Papillomaviruses and Epstein–Barr Virus Interactions in Colorectal Cancer: A Brief Review 1,2, 1,2, 1, 1,2, Queenie Fernandes y, Ishita Gupta y, Semir Vranic * and Ala-Eddin Al Moustafa * 1 College of Medicine, QU Health, Qatar University, Doha 2713, Qatar; [email protected] (Q.F.); [email protected] (I.G.) 2 Biomedical Research Centre, Qatar University, Doha 2713, Qatar * Correspondence: [email protected] (S.V.); [email protected] (A.-E.A.M.); Tel.:+974-4403-7873 (S.V.); +974-4403-7817 (A.-E.A.M.) Both authors contributed equally to this review. y Received: 9 March 2020; Accepted: 7 April 2020; Published: 20 April 2020 Abstract: Human papillomaviruses (HPVs) and the Epstein–Barr virus (EBV) are the most common oncoviruses, contributing to approximately 10%–15% of all malignancies. Oncoproteins of high-risk HPVs (E5 and E6/E7), as well as EBV (LMP1, LMP2A and EBNA1), play a principal role in the onset and progression of several human carcinomas, including head and neck, cervical and colorectal. Oncoproteins of high-risk HPVs and EBV can cooperate to initiate and/or enhance epithelial-mesenchymal transition (EMT) events, which represents one of the hallmarks of cancer progression and metastasis. Although the role of these oncoviruses in several cancers is well established, their role in the pathogenesis of colorectal cancer is still nascent. This review presents an overview of the most recent advances related to the presence and role of high-risk HPVs and EBV in colorectal cancer, with an emphasis on their cooperation in colorectal carcinogenesis. -
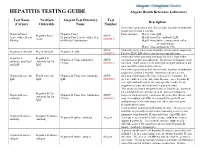
HEPATITIS TESTING GUIDE Alegent Health Reference Laboratory
HEPATITIS TESTING GUIDE Alegent Health Reference Laboratory Test Name NextGen Alegent Test Directory Test Description (Cerner) Orderable Name Number Order when patient has had clinical acute hepatitis of unknown origin for less than 6 months. Hepatitis Panel, Hepatitis Panel Panel includes; Hep A virus, IgM, Hepatitis Panel ARUP Acute with reflex to Hepatitis Panel, Acute with reflex Hep B virus Core antibody, IgM, Acute (0020457) HBsAg to HBsAg Confirmation Hep B virus Surface antigen with reflex to confirmation, Hep C virus antibody by CIA ARUP Order this assay when acute Hepatitis A infection is suspected. Hepatitis A Ab IgM Hep A Ab IgM Hepatitis A, IgM (0020093) Positive HAV IgM shows current or recent infection. Order only when assessing immunity for HAV from either Hepatitis A Hepatitis A Hepatitis A Virus Antibodies ARUP vaccination or previous infection. Do not use to diagnose acute antibody, total (IgG Antibody IgG & (Total) (0020591) infection. Total assay detects both IgG and IgM antibodies but and IgM IgM does not differentiate between them. Order when patient has had clinical acute hepatitis of unknown origin for less than 6 months. Positivity indicates recent Hepatitis B core Ab Hep B Core Ab Hepatitis B Virus Core Antibody, ARUP infection with hepatitis B virus, with onset < 6 months. It's IgM IgM IgM (0020092) presence indicates acute infection. In some cases, hepatitis B core IgM antibody may be the only specific marker for the diagnosis of acute infection with hepatitis B virus. This assay does not distinguish between Total B core antibody IgG and IgM detected before or at the onset of symptoms; Hepatitis B Core Hepatitis B core Hepatitis B Virus Core Antibodies ARUP however, such reactivity can persist for years after illness, and Antibody IgG & antibody (Total) (0020091) may even outlast anti-HBs.