Investigating the Correlates of Weak Central Coherence in Autism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bioplausible Multiscale Filtering in Retino-Cortical Processing As A
Brain Informatics (2017) 4:271–293 DOI 10.1007/s40708-017-0072-8 Bioplausible multiscale filtering in retino-cortical processing as a mechanism in perceptual grouping Nasim Nematzadeh . David M. W. Powers . Trent W. Lewis Received: 25 January 2017 / Accepted: 23 August 2017 / Published online: 8 September 2017 Ó The Author(s) 2017. This article is an open access publication Abstract Why does our visual system fail to reconstruct Differences of Gaussians and the perceptual interaction of reality, when we look at certain patterns? Where do Geo- foreground and background elements. The model is a metrical illusions start to emerge in the visual pathway? variation of classical receptive field implementation for How far should we take computational models of vision simple cells in early stages of vision with the scales tuned with the same visual ability to detect illusions as we do? to the object/texture sizes in the pattern. Our results suggest This study addresses these questions, by focusing on a that this model has a high potential in revealing the specific underlying neural mechanism involved in our underlying mechanism connecting low-level filtering visual experiences that affects our final perception. Among approaches to mid- and high-level explanations such as many types of visual illusion, ‘Geometrical’ and, in par- ‘Anchoring theory’ and ‘Perceptual grouping’. ticular, ‘Tilt Illusions’ are rather important, being charac- terized by misperception of geometric patterns involving Keywords Visual perception Á Cognitive systems Á Pattern lines -

Ibn Al-‐Haytham the Man Who Discovered How We
IBN AL-HAYTHAM THE MAN WHO DISCOVERED HOW WE SEE IBN AL-HAYTHAM EDUCATIONAL WORKSHOPS 1 Ibn al-Haytham was a pioneering scientific thinker who made important contributions to the understanding of vision, optics and light. His methodology of investigation, in particular using experiment to verify theory, shows certain similarities to what later became known as the modern scientific method. 2 Themes and Learning Objectives The educational initiative "1001 Inventions and the World of Ibn Al-Haytham" celebrates the legacy of Ibn al-Haytham. The global initiative was launched by 1001 Inventions in partnership with UNESCO in 2015 in celebration of the United Nations International Year of Light. The initiative engaged audiences around the world with events at the UNESCO headquarters in Paris, United Nations in New York, the China Science Festival in Beijing, the Royal Society in London and in more than ten other cities around the world. Inspired by Ibn al-Haytham, exhibits, hands-on workshops, science demonstrations, films and learning materials take children on a fascinating journey into the past, sparking their interest in science while promoting integration and intercultural appreciation. This document includes a range of hands-on workshops and science demonstrations, with links to fantastic resources, for understanding the fundamental principles of light, optics and vision. The activities help engage young people to make, design and tinker while better understanding the significant contributions of Ibn al-Haytham to our understanding of both vision and light. Learning Objectives ü Inspire young people to study science, technology, engineering and maths (STEM) and pursue careers in science. ü Improve awareness of light, optics and vision through Ibn al-Haytham’s discoveries. -

Modeling and Rendering of Impossible Figures
Modeling and Rendering of Impossible Figures TAI-PANG WU The Chinese University of Hong Kong CHI-WING FU Nanyang Technological University SAI-KIT YEUNG The Hong Kong University of Science and Technology JIAYA JIA The Chinese University of Hong Kong and CHI-KEUNG TANG The Hong Kong University of Science and Technology 13 This article introduces an optimization approach for modeling and rendering impossible figures. Our solution is inspired by how modeling artists construct physical 3D models to produce a valid 2D view of an impossible figure. Given a set of 3D locally possible parts of the figure, our algorithm automatically optimizes a view-dependent 3D model, subject to the necessary 3D constraints for rendering the impossible figure at the desired novel viewpoint. A linear and constrained least-squares solution to the optimization problem is derived, thereby allowing an efficient computation and rendering new views of impossible figures at interactive rates. Once the optimized model is available, a variety of compelling rendering effects can be applied to the impossible figure. Categories and Subject Descriptors: I.3.3 [Computer Graphics]: Picture/Image Generation; I.4.8 [Image Processing and Computer Vision]: Scene Analysis; J.5 [Computer Applications]: Arts and Humanities General Terms: Algorithms, Human Factors Additional Key Words and Phrases: Modeling and rendering, human perception, impossible figure, nonphotorealistic rendering ACM Reference Format: Wu, T.-P., Fu, C.-W., Yeung, S.-K., Jia, J., and Tang, C.-K. 2010. Modeling and rendering of impossible figures. ACM Trans. Graph. 29, 2, Article 13 (March 2010), 15 pages. DOI = 10.1145/1731047.1731051 http://doi.acm.org/10.1145/1731047.1731051 1. -

BYU Museum of Art M. C. Escher: Other Worlds Education Resources
BYU Museum of Art M. C. Escher: Other Worlds Education Resources Escher Terminology Crystallography: A branch of science that examines the structures and properties of crystals; greatly influenced the development of Escher’s tessellations de Mesquita, S. J.: A master printmaker at the School for Architecture and Decorative Arts, where Escher was studying, who encouraged Escher to pursue art rather than architecture H. S. M. Coxeter: A mathematician whose geometric “Coxeter Groups” became known as tessellations. Like Escher, Coxeter also loved music and its mathematical properties. The initial ideas for Circle Limit came from Coxeter. Infinity/Droste Effect: An image appearing within itself in smaller and smaller versions on to infinity; for example, when one mirror placed in front and one behind, creating an in infinite tunnel of the image Lithograph: A printing process in which the image to be printed is drawn on a flat stone surface and treated to hold ink while the negative space is treated to repel ink. Mezzotint: A method of engraving a copper or steel plate by scraping and burnishing areas to produce effects of light and shadow. Möbius strips: A surface that has only one side and one edge, making it impossible to orient; often used to symbolize eternity; used to how red ants is Escher’s Möbius Strip II Necker cube: An optical illusion proposed by Swiss crystallographer Louis Albert Necker, where the drawing of a cube has no visual cues as to its orientation; in Escher’s Belvedere, the Necker cube becomes an “impossible” cube. 1 -

1 Drawing Conclusions
Drawing Conclusions: An Exploration of the Cognitive and Neuroscientific Foundations of Representational Drawing Rebecca Susan Chamberlain Research Department of Clinical, Educational and Health Psychology Thesis submitted to UCL for the degree of Doctor of Philosophy July 2013 1 Declaration ‘I, Rebecca Susan Chamberlain confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis.’ 2 Publications The work in this thesis gave rise to the following publications: Chamberlain, R., McManus, I. C., Riley. H., Rankin, Q., & Brunswick, N. (2013) Local processing enhancements in superior observational drawing are due to enhanced perceptual functioning, not weak central coherence. Quarterly Journal of Experimental Psychology, 66 (7), 1448-66. Chamberlain, R. (2012) Attitudes and Approaches to Observational Drawing in Contemporary Artistic Practice. Drawing Knowledge. TRACEY Drawing and Visualisation Research. Chamberlain, R., McManus, I. C., Riley. H., Rankin, Q., & Brunswick, N. (In Press) Cain’s House Revisited and Revived: Extending Theory and Methodology for Quantifying Drawing Accuracy. Psychology of Aesthetics, Creativity and the Art. 3 Acknowledgements My first thanks extend to my supervisor and mentor, Professor Chris McManus, for his infectious enthusiasm for research and for the countless words of wisdom (academic and non-academic) he has given me over the last six years. I would like to thank my collaborators at The Royal College of Art and Swansea Metropolitan University, Howard Riley and Qona Rankin, who have been so generous with their time, resources and thoughts on the artistic mind. This research also wouldn’t have been possible without the hundreds of art and design students who were willing to take time out of their hectic lives to participate in my studies and discuss their experiences and reflections with me. -
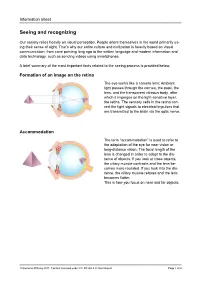
Seeing and Recognizing
Information sheet Seeing and recognizing Our society relies heavily on visual perception. People orient themselves in the world primarily us- ing their sense of sight. That’s why our entire culture and civilization is heavily based on visual communication, from cave painting long ago to the written language and modern information and data technology, such as sending videos using smartphones. A brief summary of the most important facts related to the seeing process is provided below. Formation of an image on the retina The eye works like a camera lens: Ambient light passes through the cornea, the pupil, the lens, and the transparent vitreous body, after which it impinges on the light-sensitive layer, the retina. The sensory cells in the retina con- vert the light signals to electrical impulses that are transmitted to the brain via the optic nerve. Accommodation The term “accommodation” is used to refer to the adaptation of the eye for near vision or long-distance vision. The focal length of the lens is changed in order to adapt to the dis- tance of objects: If you look at close objects, the ciliary muscle contracts and the lens be- comes more rounded. If you look into the dis- tance, the ciliary muscle relaxes and the lens becomes flatter. This is how you focus on near and far objects. © Siemens Stiftung 2017. Content licensed under CC BY-SA 4.0 international Page 1 of 4 Information sheet Seeing colors and brightness levels The retina is the light-sensitive layer of the eye containing the rod and cone cells. -

Illusion -- from Mathworld an Illusion Object Or Drawing Which Appears to Have Properties Which Are Physically Impossible, Deceptive, Or Counterintuitive
Illusion -- From MathWorld An illusion object or drawing which appears to have properties which are physically impossible, deceptive, or counterintuitive. Kitaoka maintains a web page of beautiful optical illusions which appear to show rotation despite actually being http://mathworld.wolfram.com/Illusion.html - 21k - 2003-10-05 Young Girl-Old Woman Illusion -- From MathWorld A famous perceptual illusion in which the brain switches between seeing a young girl and an old woman (or "wife" and "mother in law"). An anonymous German postcard from 1888 (left figure) depicts the image in its earliest known form, and a rendition on an advertisement for the Anchor Buggy Company from 1 http://mathworld.wolfram.com/YoungGirl-OldWomanIllusion.html - 19k - 2002-08-18 Impossible Fork -- From MathWorld ImpossibleFork.gifThe impossible fork (Seckel 2002, p. 151), also known as the devil's pitchfork (Singmaster) or blivet, is a classic impossible figure originally due to Schuster (1964). While each prong of the fork (or, in the original work, "clevis") appears normal, attempting to determine their manner http://mathworld.wolfram.com/ImpossibleFork.html - 19k - 2004-10- 01 Penrose Stairway -- From MathWorld An impossible figure in which a stairway in the shape of a square appears to circulate indefinitely while still possessing normal steps (Penrose and Penrose 1958). The Dutch artist M. C. Escher included a Penrose stairway in his mind-bending illustration "Ascending and Descending" (Bool et al. 1982, p. 3 http://mathworld.wolfram.com/PenroseStairway.html - 20k - 2004- 02-01 Hering Illusion -- From MathWorld An optical illusion due to the physiologist Ewald Hering (1861). The two horizontal lines are both straight, but they look as if they were bowed outwards. -

Constructing Drawings of Impossible Figures with Axonometric Blocks and Pseudo-3D Manipulations
Proceedings of Bridges 2014: Mathematics, Music, Art, Architecture, Culture Constructing Drawings of Impossible Figures with Axonometric Blocks and Pseudo-3D Manipulations Tiffany C. Inglis David R. Cheriton School of Computer Science University of Waterloo [email protected] Abstract Impossible figures are found in graphical designs, mathematical art, and puzzle games. There are various techniques for constructing these figures both in 2D and 3D, but most involve tricks that are not easily generalizable. We describe a simple framework that uses axonometric blocks for construction and permits pseudo-3D manipulations even though the figure may not have a real 3D counterpart. We use this framework to create impossible figures with complex structures and decorative patterns. Introduction Impossible figures [1, 6] are optical illusions involving objects that can be drawn in 2D but are infeasible in 3D (i.e., cannot be physically constructed). Figure 1a shows the impossible cube and the Penrose triangle, both of which have geometric requirements that are impossible to satisfy in 3D, and the blivet, which relies on negative space to create an illusion. Impossible figures are also featured in many of M. C. Escher’s lithographs [7], such as the Penrose stairs—an ever-ascending staircase—that is featured in both Ascending and Descending and Waterfall. Many of these impossible figures resemble 3D objects that can be constructed out of blocks under parallel projec- tion. It seems natural that one should be able to apply the same block manipulations used to construct real 3D objects to construct these impossible figures. However, since impossible figures have no 3D counterparts, defining a set of pseudo-3D manipulations is non-trivial. -

Cognitive Science
Agenda Pomona College Upcoming talks LCS 11: Cognitive Science Lorraine Tyler Thursday at 4:15PM, Edmunds 101 Vision William Marslen-Wilson Friday at noon, Edmunds 101 The eye and the visual system HoUman’s visual rules Jesse A. Harris GQ 5.1 due Sunday, 9PM April 17, 2013 Signup for group meetings Jesse A. Harris: LCS 11: Cognitive Science, Vision 1 Jesse A. Harris: LCS 11: Cognitive Science, Vision 2 The problem But this problem is nothing compared to . Vision feels easy, but it’s not at all. The miracle of vision! The visual system transforms a fragmented 2D image into a Constraints on vision coherent 3D construct. 1. Limited perceptual Veld 2. Physical blindspots 3. Subject to all kinds of illusions and perceptual error Miracle starts with the eye itself . Jesse A. Harris: LCS 11: Cognitive Science, Vision 3 Jesse A. Harris: LCS 11: Cognitive Science, Vision 4 Visible eye Cornea Curved transparent lens through which light enters. Focuses light onto the retina. Light bent and slowed by cornea. Iris Provides an adjustable aperture (colored part of eye). Pupil Hole in middle of iris, adjusts for amount of light Smaller when more light, limiting the amount let through to another lens. Jesse A. Harris: LCS 11: Cognitive Science, Vision 5 Jesse A. Harris: LCS 11: Cognitive Science, Vision 6 Inner eye Lens Contracts to give give more refractive power, needed for focusing on close objects. Power to accommodate by contraction decreases with age. Retina Light sensitive layer lining back of eye, containing multiple types of photoreceptors (rods and cones) and a chain of cells that provide the Vrst, rough processing, ending in the retinal ganglion cells. -

Optical Illusions and Their Causes Examining Differing Explanations
Optical Illusions and their Causes Examining Differing Explanations Sarah Oliver AHS Capstone 9 May, 2006 Illusion is the first of all pleasures. Oscar Wilde Introduction Optical illusions stimulate us by challenging us to see things in a new way. They are interesting within scientific disciplines because they lie on the border of what we are able to see. According to Al Seckel (2000), they “…are very useful tools for vision scientists, because they can reveal the hidden constraints of the perceptual system in a way that normal perception does not.” Different kinds of optical illusions may be better explained by one discipline or another. Some optical illusions trick us because of the properties of light and the way our eyes work, and are addressed by biology and perhaps optics, while other illusions depend on a “higher” level of processing which is better addressed by psychology. Some illusions can only be fully explained using knowledge of more than one discipline. When there are both biological and psychological explanations, they may both be right. There is already a substantial body of work that specifically addresses optical illusions, however, there is still enough disagreement within disciplines and between disciplines to make this an interesting project. One of the challenges of this project is the question of how to choose a satisfactory definition of “optical illusion.” Every source has a different definition that includes or excludes phenomena as it suits the author’s purpose. Gregory, one of the most well-known researchers of optical illusions, notes that illusion 1 … may be the departure from reality, or from truth; but how are these to be defined? As science’s accounts of reality get ever more different from appearances, to say that this separation is ‘illusion’ would have the absurd consequence of implying that almost all perceptions are illusory. -

Systematic Structural Analysis of Optical Illusion Art and Application in Graphic Design with Autism Spectrum Disorder
SYSTEMATIC STRUCTURAL ANALYSIS OF OPTICAL ILLUSION ART AND APPLICATION IN GRAPHIC DESIGN WITH AUTISM SPECTRUM DISORDER RELATORE :PROF. ARCH. PH.D ANNA MAROTTA CORRELATORE : ARCH. PH.D ROSSANA NETTI STUDENT:LI HAORAN SYSTEMATIC STRUCTURAL ANALYSIS OF OPTICAL ILLUSION ARTAND APPLICATION IN GRAPHIC DESIGN WITH AUTISM SPECTRUM DISORDER Abstract To explore the application of optical illusion in different fields, taking M.C. Escher's painting as an example, systematic analysis of optical illusion, Gestalt psychology, and Geometry are performed. Then, from the geometric point of view, to analyze the optical illusion formation model. Taking Fraser Spiral Illusion and Herring Illusion as examples, through the dismantling and combination of Fraser Spiral Illusion and the analysis of the spiral angle, and Herring Illusion tilt angle analysis, the structure of the graph is further explored. This results in a geometric drawing method that optimizes the optical illusion effect and applies this method to graphic design combined with the autism spectrum. Therefore, this project will remind the ordinary people and designers to pay attention to the universal design while also paying attention to extreme people through the combination of graphic design and optical illusion. That is, focus on autism spectrum disorder (ASD), provide more inclusive design and try their best to provide autism spectrum with the deserving autonomy and independence in public space. Meanwhile, making the autism spectrum “integrate” into the general public’s environment so that their abilities match the environment and improve self-care capabilities. Keywords Autism Spectrum Disorder, Inclusive Design, Graphic Design, Gestalt Psychology, Optical Illusion, Systemic Perspective Introduction 1. Systematic analysis of the content of the thesis 1.1 The purpose and the significance of study 1.1.1 Methodology with system diagram framework 2. -

Uncertainty in Visual Processes Predicts Geometrical Optical Illusions
Uncertainty in Visual Processes Predicts Geometrical Optical Illusions Cornelia Ferm¨uller1 and Henrik Malm2 1Computer Vision Laboratory Center for Automation Research Department of Computer Science Institute for Advanced Computer Studies University of Maryland College Park, MD 20742-3275 2Mathematical Imaging Group (MIG) Department of Mathematics (LTH) Lund Institute of Technology/ Lund University P.O. Box 118 S-221 00 Lund, Sweden Abstract It is proposed in this paper that many geometrical optical illusions, as well as illusory patterns due to motion signals in line drawings, are due to the statistics of visual computations. The interpretation of image patterns is preceded by a step where image features such as lines, intersections of lines, or local image movement must be derived. However, there are many sources of noise or uncertainty in the formation and processing of images, and they cause problems in the estimation of these features; in particular, they cause bias. As a result, the locations of features are perceived erroneously and the appearance of the patterns is altered. The bias occurs with any visual processing of line features; under average conditions it is not large enough to be noticeable, but illusory patterns are such that the bias is highly pronounced. Thus, the broader message of this paper is that there is a general uncertainty principle which governs the workings of vision systems, and optical illusions are an artifact of this principle. Key words: optical illusions, motion perception, bias, estimation processes, noise The support of this research by the National Science Foundation under grant IIS-00-8-1365 is gratefully acknowledged.