WHO. Adverse Events Following Administration of Vitamin A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

JMSCR Vol||08||Issue||02||Page 208-210||February 2020
JMSCR Vol||08||Issue||02||Page 208-210||February 2020 http://jmscr.igmpublication.org/home/ ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v8i2.40 Keratomalacia - A Case Report Authors Deepthi Pullepu1*, Deepthi Janga2, V Murali Krishna3 *Corresponding Author Deepthi Pullepu Department of Ophthalmology, Rangaraya Medical College, Kakinada, Andhra Pradesh, India Abstract Xerophthalmia refers to an ocular condition of destructive dryness of conjunctiva and cornea caused due to severe vitamin A deficiency. Usually affects infants, children and women of reproductive age group. It is estimated to affect about one-third of children under the age of five around the world. Keratomalacia means dry and cloudy cornea which melts and perforates, caused due to severe vitamin A deficiency. Here we describe a case of 21 year old female with neurofibromatosis type 1 presenting with keratomalacia. Keywords: Vitamin A deficiency, Xerophthalmia, Keratomalacia. Introduction blindness is a significant contributor Vitamin A is essential for the functioning of the In India, it is estimated that there are immune system, healthy growth and development approximately 6.8 million people who have vision of body and is usually acquired through diet. less than 6/60 in at least one eye due to corneal Globally, 190 million children under five years of diseases; of these, about a million have bilateral age are affected by vitamin A deficiency involvement. They suffer from an increased risk of visual Corneal blindness resulting due to this disease can impairment, illness and death from childhood be completely prevented by institution of effective infections such as measles and those causing preventive or prophylactic measures at the diarrhoea community level. -

Guidelines on Food Fortification with Micronutrients
GUIDELINES ON FOOD FORTIFICATION FORTIFICATION FOOD ON GUIDELINES Interest in micronutrient malnutrition has increased greatly over the last few MICRONUTRIENTS WITH years. One of the main reasons is the realization that micronutrient malnutrition contributes substantially to the global burden of disease. Furthermore, although micronutrient malnutrition is more frequent and severe in the developing world and among disadvantaged populations, it also represents a public health problem in some industrialized countries. Measures to correct micronutrient deficiencies aim at ensuring consumption of a balanced diet that is adequate in every nutrient. Unfortunately, this is far from being achieved everywhere since it requires universal access to adequate food and appropriate dietary habits. Food fortification has the dual advantage of being able to deliver nutrients to large segments of the population without requiring radical changes in food consumption patterns. Drawing on several recent high quality publications and programme experience on the subject, information on food fortification has been critically analysed and then translated into scientifically sound guidelines for application in the field. The main purpose of these guidelines is to assist countries in the design and implementation of appropriate food fortification programmes. They are intended to be a resource for governments and agencies that are currently implementing or considering food fortification, and a source of information for scientists, technologists and the food industry. The guidelines are written from a nutrition and public health perspective, to provide practical guidance on how food fortification should be implemented, monitored and evaluated. They are primarily intended for nutrition-related public health programme managers, but should also be useful to all those working to control micronutrient malnutrition, including the food industry. -

Abstract: a 19 Year Old Male Was Diagnosed with Vitamin a Deficiency
Robert Adam Young Abstract: A 19 year old male was diagnosed with vitamin A deficiency (VAD). Clinical examination shows conjunctival changes with central and marginal corneal ulcers. Patient history and lab testing were used to confirm the diagnosis. I. Case History 19 year old Hispanic Male Presents with chief complaint of progressive blur at distance and near in both eyes, foreign body sensation, ocular pain, photophobia, and epiphora; signs/symptoms are worse in the morning upon wakening. Started 6 months to 1 year ago, and has progressively gotten worse. Patient reports that the right eye is worse than the left eye. Ocular history of Ocular Rosacea Medical history of Hypoaldosteronism, Pernicious Anemia, and Type 2 Polyglandular Autoimmune Syndrome Last eye examination was two weeks ago at a medical center Presenting topical/systemic medications - Tobramycin TID OU, Prednisolone Acetate QD OU (has used for two weeks); Fludrocortisone (used long-term per PCP) Other pertinent info includes reports that patient cannot gain weight, although he has a regular appetite. Patient presents looking very slim, malnourished, and undersized for his age. II. Pertinent findings Entering unaided acuities are 20/400 OD with no improvement with pinhole; 20/50 OS that improves to 20/25 with pinhole. Pupil testing shows (-)APD; Pupils are 6mm in dim light, and constrict to 4mm in bright light. Ocular motilities are full and smooth with no reports of diplopia or pain. Tonometry was performed with tonopen and revealed intraocular pressures of 12 mmHg OD and 11 mmHG OS. Slit lamp examination shows 2+ conjunctival injection with trace-mild bitot spots both nasal and temporal, OU. -
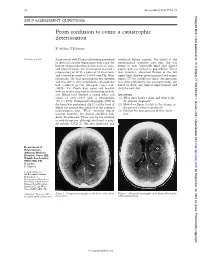
From Confusion to Coma: a Catastrophic Deterioration
52 Postgrad Med J 2001;77:52–55 Postgrad Med J: first published as 10.1136/pmj.77.903.53a on 1 January 2001. Downloaded from SELF ASSESSMENT QUESTIONS From confusion to coma: a catastrophic deterioration K Ashkan, F Johnston Answers on p 56. A previously well 45 year old woman presented ventilated before transfer. On arrival at the to the local casualty department with a one day neurosurgical intensive care unit, she was history of generalised headache, neck stiVness, found to have bilaterally fixed and dilated and blurred vision. On examination she had a pupils, with no corneal or gag reflexes. There temperature of 38°C, a pulse of 80 beats/min, was, however, abnormal flexion of the left and a blood pressure of 130/80 mm Hg. Neu- upper limb. She was given mannitol and urgent rologically, she had spontaneous eye opening repeat CT was carried out (fig 2). An operation and was able to obey commands, although she was then performed, but postoperatively she had confused speech (Glasgow coma scale failed to show any clinical improvement and (GCS), 14). Pupils were equal and reactive died the next day. with no cranial or peripheral neurological defi- cits. Blood tests showed a raised white cell Questions count of 20.9 × 109/l with a neutrophilia (1) What does figure 1 show and what is the (19.2 × 109/l). Computed tomography (CT) of diVerential diagnosis? the brain was performed (fig 1), on the basis of (2) How does figure 2 relate to the change in which the patient was referred to the regional the patient’s clinical condition? neurosurgical unit. -

Vitamins a and E and Carotenoids
Fat-Soluble Vitamins & Micronutrients: Vitamins A and E and Carotenoids Vitamins A (retinol) and E (tocopherol) and the carotenoids are fat-soluble micronutrients that are found in many foods, including some vegetables, fruits, meats, and animal products. Fish-liver oils, liver, egg yolks, butter, and cream are known for their higher content of vitamin A. Nuts and seeds are particularly rich sources of vitamin E (Thomas 2006). At least 700 carotenoids—fat-soluble red and yellow pigments—are found in nature (Britton 2004). Americans consume 40–50 of these carotenoids, primarily in fruits and vegetables (Khachik 1992), and smaller amounts in poultry products, including egg yolks, and in seafoods (Boylston 2007). Six major carotenoids are found in human serum: alpha-carotene, beta-carotene, beta-cryptoxanthin, lutein, trans-lycopene, and zeaxanthin. Major carotene sources are orange-colored fruits and vegetables such as carrots, pumpkins, and mangos. Lutein and zeaxanthin are also found in dark green leafy vegetables, where any orange coloring is overshadowed by chlorophyll. Trans-Lycopene is obtained primarily from tomato and tomato products. For information on the carotenoid content of U.S. foods, see the 1998 carotenoid database created by the U.S. Department of Agriculture and the Nutrition Coordinating Center at the University of Minnesota (http://www.nal.usda.gov/fnic/foodcomp/Data/car98/car98.html). Vitamin A, found in foods that come from animal sources, is called preformed vitamin A. Some carotenoids found in colorful fruits and vegetables are called provitamin A; they are metabolized in the body to vitamin A. Among the carotenoids, beta-carotene, a retinol dimer, has the most significant provitamin A activity. -

Hypervitaminosis - an Emerging Pathological Condition
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Review Article Hypervitaminosis - An Emerging Pathological Condition J K Roop PG Department of Zoology, JC DAV College (Affiliated to Panjab University, Chandigarh), Dasuya-144205, District Hoshiarpur, Punjab, India ABSTRACT Vitamins are essential organic compounds that are required in small amounts to regulate various metabolic activities in the body. Prolonged and overconsumption of pharmaceutical forms of both water-soluble and fat-soluble vitamins may lead to toxicity and/or hypervitaminosis. Hypervitaminosis is an acute emerging pathological condition of the body due to excess accumulation of any of the vitamins. In case of acute poisoning with vitamin supplements/drugs, emergency assistance is required to detoxify the effects and restore the organization, structure and function of body’s tissues and organs. Sometimes death may occur due to intoxication to liver, kidney and heart. So, to manage any type of hypervitaminosis, proper diagnosis is essential to initiate eliminating the cause of its occurrence and accelerate the elimination of the supplement from the body. The present review discusses the symptoms of hypervitaminosis that seems to be a matter of concern today and management strategies to overcome toxicity or hypervitaminosis. Keywords: Hypervitaminosis, Toxicity, Vitamins, Vitamin pathology, Fat-soluble vitamin, Water- soluble vitamin. INTRODUCTION body, particularly in the liver. Vitamin B Vitamins are potent organic Complex and vitamin C are water- soluble. compounds present in small concentrations They are dissolved easily in food during in various fruits and vegetables. They cooking and a portion of these vitamins may regulate physiological functions and help in be destroyed by heating. -

Current Health Issues in the Caribbean BLINDNESS in THE
Current Health Issues in the Caribbean BLINDNESS IN THE CARIBBEAN Alfred L. Anduze, M.D. St. Croix Vision Center St. Croix Hospital St. Croix U.S. Virgin Islands Caribbean Studies Association Merida, Mexico May 26, 1994 Abstract: Blindness in the Caribbean Background: The prevailing of blindness in the Caribbean region are reviewed in the context of world blindness statistics to identify differences and similarities that might exist. Method: A review of the status of blindness in the U.S. Virgin Islands, Barbados, Jamaica, Puerto Rico, Trinidad, and Mexico; individually with regard to causal etiology, epidemiology, treatment and possible future research. Results: Blindness in the Caribbean is the result of genetics, tropical environment and cultural habits of the inhabitants and consist of Age-related macular disease, Infectious diseases, Diabetes mellitus, Glaucoma, Congenital defects, Xerophthalmia, Trachoma, Trauma and Cataracts. Conclusion: There are almost 50 million people who are legally blind worldwide (i.e. with a vision of 20/200 or less) 2-3 million in the Caribbean region. The social and economic consequences are serious additional deterrents in developing countries. Outline: Causes of Blindness in the Caribbean I. Age-related macular disease a. Vascular insufficiency b. Senile macular degeneration II. Cataracts III. Glaucoma IV. Diabetes mellitus V. Infectious diseases a. Trachoma b. Onchocerciasis c. Leprosy d. Toxoplasmosis e. Toxocariasis f. AIDS VI. Trauma a. industrial/work-related b. sports c. home accidents VII. Nutritional a. Xerophthalmia/keratomalacia b. Iron-deficiency anemia c. Tobacco/Alcohol Retinopathy VIII. Congenital defects a. genetic syndromes b. strabismus Legal blindness is acceptably defined as vision 20/200 (6/60) or less. -

Question of the Day Archives: Monday, December 5, 2016 Question: Calcium Oxalate Is a Widespread Toxin Found in Many Species of Plants
Question Of the Day Archives: Monday, December 5, 2016 Question: Calcium oxalate is a widespread toxin found in many species of plants. What is the needle shaped crystal containing calcium oxalate called and what is the compilation of these structures known as? Answer: The needle shaped plant-based crystals containing calcium oxalate are known as raphides. A compilation of raphides forms the structure known as an idioblast. (Lim CS et al. Atlas of select poisonous plants and mushrooms. 2016 Disease-a-Month 62(3):37-66) Friday, December 2, 2016 Question: Which oral chelating agent has been reported to cause transient increases in plasma ALT activity in some patients as well as rare instances of mucocutaneous skin reactions? Answer: Orally administered dimercaptosuccinic acid (DMSA) has been reported to cause transient increases in ALT activity as well as rare instances of mucocutaneous skin reactions. (Bradberry S et al. Use of oral dimercaptosuccinic acid (succimer) in adult patients with inorganic lead poisoning. 2009 Q J Med 102:721-732) Thursday, December 1, 2016 Question: What is Clioquinol and why was it withdrawn from the market during the 1970s? Answer: According to the cited reference, “Between the 1950s and 1970s Clioquinol was used to treat and prevent intestinal parasitic disease [intestinal amebiasis].” “In the early 1970s Clioquinol was withdrawn from the market as an oral agent due to an association with sub-acute myelo-optic neuropathy (SMON) in Japanese patients. SMON is a syndrome that involves sensory and motor disturbances in the lower limbs as well as visual changes that are due to symmetrical demyelination of the lateral and posterior funiculi of the spinal cord, optic nerve, and peripheral nerves. -

2002 Samel 10 Most Common Systemic Health Conditions with A
Avanti Samel / March 15, 2002 10 Most Common Systemic Health Conditions with a Listing of Frequently Prescribed Medications and their Ocular Side Effects HYPERTENSION ACE Inhibitors: Captopril (Capoten}- blurred vision Enalapril (Vasotec) - Blurred vision, conjunctivitis, dry eyes, tearing Quinipril (Accupril)- amblyopia Benazepril (Lotensin)- N/A Lisinopril (Zestril) - Visual loss, diplopia, blurred vision, photophobia Beta-Blockers: Propranolol (Inderal)- visual disturbances, dry eyes Atenolol (Tenormin)- blurred vision, dry eyes, visual disturbances Metoprolol (Lopressor) - blurred vision, dry eyes calcium Channel Blockers: Diltiazem (Cardizem)- Amblyopia, eye irritation Amlodipine (Norvasc)- abnormal vision, conjunctivitis, diplopia, eye pain Verapamil (Calan)- Blurred vision Nifedipine (Procardia) - blurred vision, Transient blindness at the peak of plasma level · Diuretics: Thiazides: Chlorothiazide (Diuril) - Transient blurred vision, xanthopsia Loop: Furosemide (Lasix) - Blurred vision, Xanthopsia Potassium Sparing: Amiloride (Midamor) - Visual disturbances, Increased lOP Triamterene (Dyrenium)- N/A HYPERLIPIDEMIA Statins: Lovastatin (Mevacor) - Blurred vision, Eye irritation Simvastatin (Zocor)- Cataracts Atorvastatin (Lipitor)- Amblyopia, dry eyes, refraction disorder, eye hemorrhage, glaucoma Resins: Cholestyramine (Questran)- Uveitis Fibrates: Gemfibrozil (Lopid)- Blurred vision Niacin: Niacin (Niacor) - Toxic amblyopia, cystoid macular edema ASTHMA ( ) Beta 2 Agonists: Albuterol (Proventil) - N/A ( Salmeterol (Serevent)- -

Vitamin a Fact Sheet
Vitamin A Fact Sheet Why does the body need vitamin A? Vitamin A helps: *To maintain vision in dim light *To keep skin smooth and healthy *To help you grow *To help keep your insides healthy (inner linings of the mouth, ears, nose, lungs, urinary and digestive tract) What foods are good sources of vitamin A? Many orange and dark green vegetables and fruits contain carotenes, natural coloring substances, or pigments. The body can change these pigments into vitamin A. The deeper the green or orange color of the vegetable or the fruit, the more carotenes (and thus vitamin A) it contains. Green Vegetables - broccoli, asparagus, spinach, kale, chard, collards and beet, mustard, turnip or dandelion greens. Orange Fruits and Vegetables - carrots, winter squash, sweet potatoes, tomatoes, pumpkin, apricots, cantaloupes, nectarines, peaches, papaya and mangoes. Watch out! - Color is not always a way to recognize foods rich in vitamin A. Oranges, lemons, grapefruits or tangerines are orange or yellow in color but do not contain much carotene. Also, yams, in contrast to sweet potatoes, have no vitamin A value. Is vitamin A stored in my body? Yes, Vitamin A is stored in the liver, so a rich source of vitamin A does not have to be included in the diet every day. However, vitamin A foods included in the diet every day help to build the body reserve. This may be needed in the case of illness or any time when vitamin A is lacking in the diet. How much vitamin A do I need every day? Recommended intake based on the Dietary Reference Intakes (DRI) for Vitamin -

Dietary Supplements Compendium Volume 1
2015 Dietary Supplements Compendium DSC Volume 1 General Notices and Requirements USP–NF General Chapters USP–NF Dietary Supplement Monographs USP–NF Excipient Monographs FCC General Provisions FCC Monographs FCC Identity Standards FCC Appendices Reagents, Indicators, and Solutions Reference Tables DSC217M_DSCVol1_Title_2015-01_V3.indd 1 2/2/15 12:18 PM 2 Notice and Warning Concerning U.S. Patent or Trademark Rights The inclusion in the USP Dietary Supplements Compendium of a monograph on any dietary supplement in respect to which patent or trademark rights may exist shall not be deemed, and is not intended as, a grant of, or authority to exercise, any right or privilege protected by such patent or trademark. All such rights and privileges are vested in the patent or trademark owner, and no other person may exercise the same without express permission, authority, or license secured from such patent or trademark owner. Concerning Use of the USP Dietary Supplements Compendium Attention is called to the fact that USP Dietary Supplements Compendium text is fully copyrighted. Authors and others wishing to use portions of the text should request permission to do so from the Legal Department of the United States Pharmacopeial Convention. Copyright © 2015 The United States Pharmacopeial Convention ISBN: 978-1-936424-41-2 12601 Twinbrook Parkway, Rockville, MD 20852 All rights reserved. DSC Contents iii Contents USP Dietary Supplements Compendium Volume 1 Volume 2 Members . v. Preface . v Mission and Preface . 1 Dietary Supplements Admission Evaluations . 1. General Notices and Requirements . 9 USP Dietary Supplement Verification Program . .205 USP–NF General Chapters . 25 Dietary Supplements Regulatory USP–NF Dietary Supplement Monographs . -

Nutritional Value of Spirulina and Its Use in the Preparation of Some Complementary Baby Food Formulas
Available online at http://journal-of-agroalimentary.ro Journal of Agroalimentary Processes and Journal of Agroalimentary Processes and Technologies Technologies 2014, 20(4), 330-350 Nutritional value of spirulina and its use in the preparation of some complementary baby food formulas Ashraf M. Sharoba Food Sci. Dept., Fac. of Agric., Moshtohor, Benha Univ., Egypt Received: 26 September 2014; Accepted:03 Octomber 2014 .____________________________________________________________________________________ Abstract In this study use the spirulina which is one of the blue-green algae rich in protein 62.84% and contains a high proportion of essential amino acids (38.46% of the protein) and a source of naturally rich in vitamins especially vitamin B complex such as vitamin B12 (175 µg / 10 g) and folic acid (9.92 mg / 100 g), which helps the growth and nutrition of the child brain, also rich in calcium and iron it containing (922.28 and 273.2 mg / 100 g, respectively) to protect against osteoporosis and blood diseases as well as a high percentage of natural fibers. So, the spirulina is useful and necessary for the growth of infants and very suitable for children, especially in the growth phase, the elderly and the visually appetite. It also, helps a lot in cases of general weakness, anemia and chronic constipation. Spirulina contain an selenium element (0.0393 mg/100 g) and many of the phytopigments such as chlorophyll and phycocyanin (1.56% and 14.647%), and those seen as a powerful antioxidant. Finally, spirulina called the ideal food for mankind and the World Health Organization considered its "super food" and the best food for the future because of its nutritional value is very high.