Acrolein Hazards to Fish, Wildlife, and Invertebrates: a Synoptic Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pre-Validation of an Acute Inhalation Toxicity Assay Using the Epiairway in Vitro Human Airway Model
Pre-Validation of an Acute Inhalation Toxicity Assay Using the EpiAirway In Vitro Human Airway Model George R. Jackson, Jr., Michelle Debatis, Anna G. Maione, Patrick J. Hayden Exposure to potentially dangerous chemicals can occur through inhalation. UNDERSTANDING HUMAN BIOLOGY IN DIMENSIONS3 2 Regulatory systems for classifying the acute inhalation toxicity of chemicals ≤ 0.05 mg/l > 0.05 ≤ 0.5 mg/l > 0.5 ≤ 2 mg/l > 2 mg/l Respirator Use Required 3 Regulatory systems for classifying the acute inhalation toxicity of chemicals 4 OECD 403/436 is the currently accepted test method for determining acute inhalation toxicity OECD Test Guidelines 403/436: In vivo rat LD50 test (dose at which 50% of the animals die) 4 hour exposure 14 Days Examination: - Death -Signs of toxicity -Necropsy should be performed (not always reported) Nose/Head only (preferred) Whole body Repeat stepwise with additional concentrations as necessary 5 Our goal is to develop & validate an in vitro test for acute inhalation toxicity UNDERSTANDING HUMAN BIOLOGY IN DIMENSIONS3 6 The EpiAirway Model EpiAirway is an in vitro 3D organotypic model of human tracheal/bronchial tissue. - Constructed from primary cells - Highly reproducible - Differentiated epithelium at the air-liquid interface - Beating cilia - Mucus secretion - Barrier function - Physiologically relevant & predictive of the human outcome Air Cilia Differentiated epithelium Microporous membrane Media 7 EpiAirwayTM acute inhalation toxicity test method Prepare 4-point dose Apply chemical to Incubate for 3 hours Examination: curve of chemical in the apical surface - Tissue viability (MTT) dH2O or corn oil Advantages of using the in vitro EpiAirway test: 1. -

1997-11-12 Acrolein As Federal Hazardous Air Pollutant
ACROLEIN Acrolein is a federal hazardous air pollutant and was identified as a toxic air contaminant in April 1993 under AB 2728. CAS Registry Number: 107-02-8 H2C=CHCHO Molecular Formula: C3H4O Acrolein is a colorless or yellowish, flammable liquid with an unpleasant, extremely pungent odor. It is soluble in petroleum ether, water, and alcohol and miscible with hydrocarbons, acetone, and benzene (Sax, 1989). Acrolein polymerizes (especially in the presence of light, alkali, or strong acid) forming disacryl, a plastic solid (Merck, 1989). Physical Properties of Acrolein Synonyms: acraldehyde; allyl aldehyde; acrylic aldehyde; Biocide; 2-propenal Molecular Weight: 56.06 Boiling Point: 52.5 oC Melting Point: -88.0 oC Flash Point: -18 oC (< 0 oF) (open cup) Vapor Density: 1.94 (air = 1) Vapor Pressure: 210 mm Hg at 20 oC Density/Specific Gravity: 0.8389 at 20/4 oC Log Octanol/Water Partition Coefficient: -0.09 Water Solubility: 208,000 mg/L at 20 oC Henry's Law Constant: 4.4 x 10-6 atm-m3/mole Conversion Factor: 1 ppm = 2.29 mg/m3 (Howard, 1990; HSDB, 1991; Merck, 1989; U.S. EPA, 1994a) SOURCES AND EMISSIONS A. Sources Acrolein is emitted from sources where it is manufactured and used as an intermediate for glycerine, methionine, glutaraldehyde, and other organic chemicals. It is also found in tobacco smoke, forest fire emissions, and gasoline and diesel exhaust. Acrolein is also a photooxidation product of various hydrocarbons including 1,3-butadiene (Howard, 1990). Toxic Air Contaminant Identification List Summaries - ARB/SSD/SES September 1997 23 Acrolein The primary stationary sources that have reported emissions of acrolein in California are paper mills, and abrasive, asbestos, miscellaneous non-metallic mineral, and wood products (ARB, 1997b). -

Filter Chart
Filter Chart Chemical Filter Chemical Filter Chemical Filter Abate FFP1 tert-Butyl acetate A1 Copper fume FFP1 Acetaldehyde A1 Butyl acrylate A1 Dusts & mist (as Cu) FFP1 Acetic acid E1 n-Butyl alcohol A1 Cotton dust, raw FFP1 Acetic anhydride B1 sec-Butyl alcohol R A1 Crag herbicide FFP1 Acetonitrile A1 Butylamine B1 Cresol, all isomers FFP1 Acetylene dichloride A1 tert-Butyl chromate (as Cro3) FFP1 Cumene FFP1 Acetylene tetrabromide A1 n-Butyl glycidyl ether(BGE) A1 Cyanamide A1 P1 Acetylsalicylic acid FFP2 n-Butyl lactate A1 Cyanogen B1 Acrolein A1 o-sec Butyl phenol A1 Acrylamide A1 P2 p-tert Butyltoluene A1 Cyanogen chloride (CK) B1 Acrylonitrile A1 Cadmium, dust & salts (as Cd) FFP1 Cyclohexane A1 Aldrin A1 P2 Cadmium oxide fume (as Cd) FFP1 Cyclohexnol A1 Allyl alcohol A1 Calcium cyanamide FFP1 Cyclohexanone A1 Allyl chloride A1 Calcium hydroxide FFP1 Cyclohexene Allyl glycidyl ether (AGE) A1 Calcium oxide FFP1 A1 Allyl propyl disulfide B1 Camphor, synthetic A1 Cyclohexylamine A1 Aluminium metal and oxide FFP2 Caprolactam Dust FFP1 Cyclonite B1 Aluminium pyro powders FFP2 Vapor A1 1.3 Cyclopentadiene A1 Aluminium welding fumes A1 P2 Captafol(DifolatanR) FFP1 2.4-D (2.4-Dichlorophenoxy acetic acid) FFP1 Aluminium soluble salts FFP2 Captan FFP1 Aluminium, alkyls A1 R Carbary (Seven ) FFP1 D.D.T. (Dichlorodiphenyl A1 P1 4-Aminodiphenyl FFP1 Carbofuran (FuradanR) FFP1 trichloroethane) A1 P1 2- Aminoethanol A1 Carbon black FFP1 DDVP Decaborane B1 P1 2- Aminopyridine K1 Carbon dusulfide B1 DemetonR B1 P1 Ammonia A1 Carbon tetrabromide -

Acrolein Is Absorbed; Have Been Identified for Acrolein
TM The ToxGuide is developed to be used as a pocket guide. Tear off at perforation and fold along lines. Toxicokinetics and Biomarkers/Environmental Sources of Exposure Normal Human Levels Levels ToxGuideTM General Populations Toxicokinetics Biomarkers Exposure may occur through inhalation, Based on animal data, approximately No biomarkers of exposure or effect for ingestion, and dermal contact. 80–90% of inhaled acrolein is absorbed; have been identified for acrolein. Environmental tobacco smoke (ETS) is most in the upper respiratory tract. the primary source of exposure for many In vitro studies suggest that acrolein will Environmental Levels individuals. form conjugates with glutathione. Air Acrolein Widespread exposure occurs due to the Following oral exposure in animals, formation of acrolein during the heating approximately 30% of the initial dose is Acrolein levels in outdoor air averaged from 0.5 to 3.186 ppb. of fats. expired as carbon dioxide and 50-60% is CH2=CH-CHO The general population may also be excreted in the urine. Acrolein in indoor air ranged from <0.02 to 12 ppb in residential homes. exposed to high concentrations from CAS# 107-02-8 vehicle exhaust (for example, parking Normal Human Levels Sediment and Soil October 2007 garages and/or heavy traffic). No data available No data are available on actual Acrolein is also present in certain foods measurements of acrolein in soil. such as raw cocoa beans, chocolate Water U.S. Department of Health and liquor, fried potatoes and onions, raw and Acrolein has not been found as a Human Services cooked turkey, heated animal fats and contaminant of drinking water. -

Toxicological Profile for Acrolein
ACROLEIN 1 1. PUBLIC HEALTH STATEMENT This public health statement tells you about acrolein and the effects of exposure to it. The Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in the nation. These sites are then placed on the National Priorities List (NPL) and are targeted for long-term federal clean-up activities. Acrolein has been found in at least 32 of the 1,684 current or former NPL sites. Although the total number of NPL sites evaluated for this substance is not known, the possibility exists that the number of sites at which acrolein is found may increase in the future as more sites are evaluated. This information is important because these sites may be sources of exposure and exposure to this substance may harm you. When a substance is released either from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment. Such a release does not always lead to exposure. You can be exposed to a substance only when you come in contact with it. You may be exposed by breathing, eating, or drinking the substance, or by skin contact. If you are exposed to acrolein, many factors will determine whether you will be harmed. These factors include the dose (how much), the duration (how long), and how you come in contact with it. You must also consider any other chemicals you are exposed to and your age, sex, diet, family traits, lifestyle, and state of health. 1.1 WHAT IS ACROLEIN? Acrolein is a clear or yellow liquid with a burnt, sweet, pungent odor. -

Determination of Acetaldehydes and Acrolein in the Gas Phase of Cigarette Smoke Using Cryothermal Gas Cromatography’
DETERMINATION OF ACETALDEHYDES AND ACROLEIN IN THE GAS PHASE OF CIGARETTE SMOKE USING CRYOTHERMAL GAS CROMATOGRAPHY’ By A. D. HORTON and M. R. GUERIN Analytical Chemistry Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee, U.S.A. 37830 Tobacco Science, 1974, 18-8, p. 19-22, ISSN.0082-4523.pdf Acetaldehyde and acrolein in cigarette smoke have been de- faitt ;I ~I~IX’acroleiti clution peak. termined by gas chromatography of the gas phase which has Ott<, 01’ t ht, more successful methods ol’ cryotherm:tl been trapped at -75 ‘C on the head of the column in a cryo- 1t ’;tihItittp 1~~s developed by Laurette, L~erly and Young thermal gas chromatograph. A system which allows standard mix- tures to be sampled in the same manner as the cigarette has 1X I \\,ho t IX~IJK~ gas phaw in it copper “radiator” trap been developed. By use of this system, quantitative acetaldehyde intntersc~tl i tt liquid nitrogen. The trap wlrws~pumped at and acrolein values were assigned to a reference cigarette, which Iiquitl ttitt’o~i’tt temperature to r~~rnove noticot~detisible was used as a secondary standard. For twenty-five experimental cigarettes? the average acetatdehyde [:ontent ranged from 681 to g’;tst-‘. ;lttd thr contents \VPIY transferwd to the gas 1309,Lgiclg., and acrolein ranged from 59 to 139 Ug!cig.. with ~ht~ont;~tl)~t.~t~)ll,VIA ;L gas sampling valve, by heating an average coefficient of variation of 2.69, for acetaldehyde and 2.8% for acrotein. The Cl,,r, for acetnldehyde was 6.5% and for iht, 11’;11) it1 2111 oil bath. -

The Chemistry of 3-Acetyl-3, 4-Phenacylidenecoumarin Gerald Eugene Risinger Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1961 The chemistry of 3-acetyl-3, 4-phenacylidenecoumarin Gerald Eugene Risinger Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Risinger, Gerald Eugene, "The chemistry of 3-acetyl-3, 4-phenacylidenecoumarin " (1961). Retrospective Theses and Dissertations. 1983. https://lib.dr.iastate.edu/rtd/1983 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been 62-1367 microfilmed exactly as received RISINGER, Gerald Eugene, 1932- THE CHEMISTRY OF 3-ACETYL-3,4-PHENACYLI- DENECOUMARIN. Iowa State University of Science and Technology Ph.D., 1961 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan THE CHEMISTRY OF 3-ACETYL-3 , ^-PI-ENACYLIDENECOUKARIN by Gerald Eugene Risinger A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject : Organic Chemistry Approved: Signature was redacted for privacy. In Charge of Major Work Signature was redacted for privacy. head of Major Department Signature was redacted for privacy. Iowa State University Of Science and Technology Ames, Iowa 1961 il TABLE OF CONTENTS PAGE I INTRODUCTION 1 II HISTORICAL 2 III DISCUSSION 49 IV SPECTRA 87 V EXPERIMENTAL 100 VI SUMMARY 116 VII LITERATURE CITED . -

Differences in Exposure to Nicotine, Tobacco-Specific Nitrosamines
toxics Article Differences in Exposure to Nicotine, Tobacco-Specific Nitrosamines, and Volatile Organic Compounds among Electronic Cigarette Users, Tobacco Smokers, and Dual Users from Three Countries Danielle M. Smith 1 , Lion Shahab 2 , Benjamin C. Blount 3 , Michal Gawron 4, Leon Kosminder 4 , Andrzej Sobczak 4, Baoyun Xia 3, Connie S. Sosnoff 3 and Maciej L. Goniewicz 1,* 1 Department of Health Behavior, Roswell Park Comprehensive Cancer Center, Elm and Carlton Streets, Buffalo, NY 14263, USA; [email protected] 2 Department of Epidemiology and Public Health, University College London, 1-19 Torrington Place, London WC1E 6BT, UK; [email protected] 3 Tobacco and Volatiles Branch, Division of Laboratory Sciences, Centers for Disease Control and Prevention, Atlanta, GA 30341, USA; [email protected] (B.C.B.); [email protected] (B.X.); [email protected] (C.S.S.) 4 Department of General and Inorganic Chemistry, Katowice Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Jagiellonska 4, 41-200 Sosnowiec, Poland; [email protected] (M.G.); [email protected] (L.K.); [email protected] (A.S.) * Correspondence: [email protected]; Tel.: +1-716-845-8541; Fax: +1-716-845-1268 Received: 24 August 2020; Accepted: 12 October 2020; Published: 14 October 2020 Abstract: Country-level differences in nicotine vaping products used and biomarkers of exposure among long-term e-cigarette users and dual users remain understudied. This cross-sectional study was conducted in 2014 in the United States (n = 166), United Kingdom (n = 129), and Poland (n = 161). We compared patterns of tobacco product use and nicotine and toxicant exposure among cigarette-only smokers (n = 127); e-cigarette-only users (n = 124); dual users of tobacco cigarettes and e-cigarettes (n = 95); and non-users (control group, n = 110) across three countries using mixed-effects linear regression. -

Triacetin Enhances Levels of Acrolein, Formaldehyde Hemiacetals, and Acetaldehyde in Electronic Cigarette Aerosols
Portland State University PDXScholar Chemistry Faculty Publications and Presentations Chemistry 7-2018 Triacetin Enhances Levels of Acrolein, Formaldehyde Hemiacetals, and Acetaldehyde in Electronic Cigarette Aerosols Shawna Vreeke Portland State University David H. Peyton Portland State University, [email protected] Robert Strongin Portland State University, [email protected] Follow this and additional works at: https://pdxscholar.library.pdx.edu/chem_fac Part of the Chemistry Commons Let us know how access to this document benefits ou.y Citation Details Vreeke, S., Peyton, D. H., & Strongin, R. M. (2018). Triacetin Enhances Levels of Acrolein, Formaldehyde Hemiacetals, and Acetaldehyde in Electronic Cigarette Aerosols. ACS omega, 3(7), 7165-7170. This Article is brought to you for free and open access. It has been accepted for inclusion in Chemistry Faculty Publications and Presentations by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes. Article Cite This: ACS Omega 2018, 3, 7165−7170 Triacetin Enhances Levels of Acrolein, Formaldehyde Hemiacetals, and Acetaldehyde in Electronic Cigarette Aerosols Shawna Vreeke, David H. Peyton, and Robert M. Strongin* Department of Chemistry, Portland State University, 1719 SW 10th Avenue, Portland, Oregon 97201, United States *S Supporting Information ABSTRACT: The health effects of inhaled electronic cigarette (e- cigarette) flavoring compounds are largely unknown. Earlier reports of their chemical reactivity have been conflicting, with some claiming, for example, that the degradation of flavoring chemicals in e-cigarettes to aldehydes is statistically insignificant. -

Chemical Compatibility Chart
Chemical Compatibility Chart 1 Inorganic Acids 1 2 Organic acids X 2 3 Caustics X X 3 4 Amines & Alkanolamines X X 4 5 Halogenated Compounds X X X 5 6 Alcohols, Glycols & Glycol Ethers X 6 7 Aldehydes X X X X X 7 8 Ketone X X X X 8 9 Saturated Hydrocarbons 9 10 Aromatic Hydrocarbons X 10 11 Olefins X X 11 12 Petrolum Oils 12 13 Esters X X X 13 14 Monomers & Polymerizable Esters X X X X X X 14 15 Phenols X X X X 15 16 Alkylene Oxides X X X X X X X X 16 17 Cyanohydrins X X X X X X X 17 18 Nitriles X X X X X 18 19 Ammonia X X X X X X X X X 19 20 Halogens X X X X X X X X X X X X 20 21 Ethers X X X 21 22 Phosphorus, Elemental X X X X 22 23 Sulfur, Molten X X X X X X 23 24 Acid Anhydrides X X X X X X X X X X 24 X Represents Unsafe Combinations Represents Safe Combinations Group 1: Inorganic Acids Dichloropropane Chlorosulfonic acid Dichloropropene Hydrochloric acid (aqueous) Ethyl chloride Hydrofluoric acid (aqueous) Ethylene dibromide Hydrogen chloride (anhydrous) Ethylene dichloride Hydrogen fluoride (anhydrous) Methyl bromide Nitric acid Methyl chloride Oleum Methylene chloride Phosphoric acid Monochlorodifluoromethane Sulfuric acid Perchloroethylene Propylene dichloride Group 2: Organic Acids 1,2,4-Trichlorobenzene Acetic acid 1,1,1-Trichloroethane Butyric acid (n-) Trichloroethylene Formic acid Trichlorofluoromethane Propionic acid Rosin Oil Group 6: Alcohols, Glycols and Glycol Ethers Tall oil Allyl alcohol Amyl alcohol Group 3: Caustics 1,4-Butanediol Caustic potash solution Butyl alcohol (iso, n, sec, tert) Caustic soda solution Butylene -

Toxicity Factors for Camphor Ground Water Soil Surface Water Drinking
NJDEP - Toxicity Factors for Camphor These are the human health toxicity data that were used by the Department to derive its health based criteria. 76-22-2 Drinking water Carcinogen Group: Oral Slope Factor: (mg/kg/day) -1 Oral Reference Dose: (mg/kg/day) Basis: Ground water Carcinogen Group: Dc Oral Slope Factor: (mg/kg/day) -1 Oral Reference Dose: 0.18 (mg/kg/day) Basis: DEP Surface water Carcinogen Group Oral Slope Factor: (mg/kg/day)-1 Oral Reference Dose: (mg/kg/day) Basis: Soil Oral Inhalation Carcinogen Group Carcinogen Group : Slope Factor: Unit Risk Factor -1 (mg/kg/day) (ug/m3 )-1 Reference Dose: Reference (mg/kg/day) Concentration: (ug/m) 3 Basis: Basis: * Reference Doses for Group C chemicals are shown with uncertainty factor of 10 for possible carcinogenicity included. These are theReference Doses used to derive criteria for all media. In the Basis and Background documents for these criteria, these Reference Doses may or may not be shown with this uncertainty factor incorporated. New Jersey Dept. of Environmental Protection - Toxicity Factors 9/23/2008 Page 1 See additional footnote explanations on last page Drinking Water - Notes 1. The Reference Doses for the Group C chemicals incorporate an additional uncertainty factor of 10 for possible carcinogenicity. 2. Toxicity factors were developed by the NJDWQI under the A-280 process for the following chemicals, but MCLs were not adopted for unrelated reasons, such as lack of a standardized analytical method for drinking water: Ethylene glycol, formaldehyde, hexane, methyl ethyl ketone, and 2,4,6-trichlorophenol. 3. The New Jersey MCL for 1,4-Dichlorobenzene was adopted from USEPA, but New Jersey did not necessarily agree with the USEPA RfD, so it is not included on this table Ground Water - Footnotes a = from USEPA, Health Effects Assessment Summary Tables, FY 1997 Update, OSWER 9200.6.303 (97-1), EPA-540-R-97-036, PB97-921199, July 1997. -
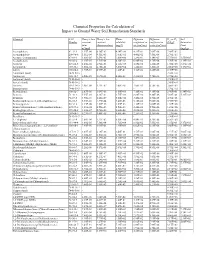
Chemical Properties Table
Chemical Properties for Calculation of Impact to Ground Water Soil Remediation Standards Chemical CAS Henry’s law Henry’s law Water Diffusion Diffusion Koc or Kd Soil Number constant constant solubility coefficient in coefficient in (L/kg)a Saturation (atm- (dimensionless) (mg/L) air (cm2/sec) water (cm2/sec) Limit m3/mol) (mg/kg) Acenaphthene 83-32-9 1.55E-04 6.36E-03 4.24E+00 4.21E-02 7.69E-06 7.08E+03 - Acenaphthylene 208-96-8 1.11E-04 4.51E-03 1.60E+01 4.40E-02 7.50E-06 2.76E+03 - Acetone (2-propanone) 67-64-1 3.88E-05 1.59E-03 1.00E+06 1.24E-01 1.14E-05 5.75E-01 1.55E+05 Acetophenone 98-86-2 1.10E-05 4.51E-04 6.10E+03 6.00E-02 8.70E-06 3.70E+01 1.39E+03 Acrolein 107-02-8 1.20E-04 4.92E-03 2.10E+05 1.05E-01 1.20E-05 1.00E+00 3.27E+04 Acrylonitrile 107-13-1 1.00E-04 4.10E-03 7.40E+04 1.22E-01 1.30E-05 2.00E+00 1.17E+04 Aldrin 309-00-2 1.70E-04 6.97E-03 1.80E-01 1.32E-02 4.86E-06 2.45E+06 - Aluminum (total) 7429-90-5 - - - - - 1.50E+03 - Anthracene 120-12-7 6.50E-05 2.67E-03 4.34E-02 3.24E-02 7.74E-06 2.95E+04 - Antimony (total) 7440-36-0 - - - - - 4.50E+01 - Arsenic (total) 7440-38-2 - - - - - 2.60E+01 - Atrazine 1912-24-9 2.96E-09 1.21E-07 7.00E+01 2.60E-02 6.70E-06 3.60E+02 - Barium (total) 7440-39-3 - - - - - 1.70E+01 - Benzaldehyde 100-52-7 2.67E-05 1.09E-03 3.00E+03 7.30E-02 9.10E-06 2.90E+01 6.34E+02 Benzene 71-43-2 5.55E-03 2.28E-01 1.75E+03 8.80E-02 9.80E-06 5.89E+01 5.22E+02 Benzidine 92-87-5 3.90E-11 1.60E-09 5.00E+02 3.40E-02 1.50E-05 4.70E+01 - Benzo(a)anthracene (1,2-Benzanthracene) 56-55-3 3.35E-06 1.37E-04 9.40E-03 5.10E-02