Introduction Louisville, KY Learning Objectives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Program PDF Saturday, March 28, 2020 Updated: 02-14-20
Program PDF Saturday, March 28, 2020 Updated: 02-14-20 Special ‐ Events and Meetings Congenital Heart Disease ‐ Scientific Session #5002 Session #602 Fellowship Administrators in Cardiovascular Education and ACHD Cases That Stumped Me Training Meeting, Day 2 Saturday, March 28, 2020, 8:00 a.m. ‐ 9:30 a.m. Saturday, March 28, 2020, 7:30 a.m. ‐ 5:30 p.m. Room S105b Marriott Marquis Chicago, Great Lakes Ballroom A CME Hours: 1.5 / CNE Hours: CME Hours: / CNE Hours: Co‐Chair: C. Huie Lin 7:30 a.m. Co‐Chair: Karen K. Stout Fellowship Administrators in Cardiovascular Education and Training Meeting, Day 2 8:00 a.m. LTGA, Severe AV Valve Regurgitation, Moderately Reduced EF, And Atrial Acute and Stable Ischemic Heart Disease ‐ Scientific Arrhythmia Session #601 Elizabeth Grier Treating Patients With STEMI: What They Didn't Teach You in Dallas, TX Fellowship! Saturday, March 28, 2020, 8:00 a.m. ‐ 9:30 a.m. 8:05 a.m. Room S505a ARS Questions (Pre‐Panel Discussion) CME Hours: 1.5 / CNE Hours: Elizabeth Grier Dallas, TX Co‐Chair: Frederick G. Kushner Co‐Chair: Alexandra J. Lansky 8:07 a.m. Panelist: Alvaro Avezum Panel Discussion: LTGA With AVVR And Reduced EF Panelist: William W. O'Neill Panelist: Jennifer Tremmel Panelist: Jonathan Nathan Menachem Panelist: Joseph A. Dearani 8:00 a.m. Panelist: Michelle Gurvitz Case of a Young Women With STEMI Panelist: David Bradley Jasjit Bhinder Valhalla, NY 8:27 a.m. ARS Questions (Post‐Panel Discussion) 8:05 a.m. Elizabeth Grier Young Women With STEMI: Something Doesn't Make Sense... -

The Increase of the Pulmonary Blood Flow Inhigh-Risk Hypoxic Patients with a Bidirectional Glenn Anastomosis
ORIGINAL ARTICLE The increase of the pulmonary blood flow inhigh-risk hypoxic patients with a bidirectional Glenn anastomosis Jacek Kołcz1, Mirosława Dudyńska2, Aleksandra Morka3, Sebastian Góreczny4, Janusz Skalski1 1Department of Pediatric Cardiac Surgery, Jagiellonian University Medical College, Kraków, Poland 2Department of Pediatric Cardiac Surgery, University Children’s Hospital in Krakow, Kraków, Poland 3Faculty of Health Sciences, Jagiellonian University Medical College, Kraków, Poland 4Department of Pediatric Cardiology, Jagiellonian University Medical College, Kraków, Poland Correspondence to: Jacek Kołcz, MD, PhD, ABSTRACT FECTS, Department of Pediatric Background: An additional shunt in single ventricle patients with Glenn anastomosis may increase Cardiac Surgery, pulmonary flow at the expense of ventricle volume overloading. The performance of the modification Jagiellonian University depends on pulmonary resistance, indicating better results in favorable hemodynamic conditions. Medical College, Wielicka 265, Aims: The study aims at analyzing the influence of precisely adjusted pulsatile shunt in borderline 30–663 Kraków, Poland, phone: +40 12 658 20 11, high-risk Glenn patients on early and late results. e-mail: Methods: The study involved 99 patients (including 21 children) with the bidirectional Glenn and ac- [email protected] cessory pulsatile shunt (BDGS group), and 78 patients with the classic bidirectional Glenn anastomosis Copyright by the Author(s), 2021 (BDG group). Kardiol Pol. 2021; Results: There was 1 death in the BDGS group and 4 deaths in the BDG group. No difference in mortality 79 (6): 638–644; DOI: 10.33963/KP.15939 (P = 0.71) was found. The Fontan completion was achieved in 69 (88.5%) children in the BDG group and Received: 18 (85.7%) patients in the BDGS group, without fatalities. -

The Physiology of the Fontan Circulation ⁎ Andrew Redington
Progress in Pediatric Cardiology 22 (2006) 179–186 www.elsevier.com/locate/ppedcard The physiology of the Fontan circulation ⁎ Andrew Redington Division of Cardiology, The Department of Pediatrics, The Hospital for Sick Children, University of Toronto School of Medicine, Toronto, Ontario, Canada Available online 8 September 2006 Abstract The Fontan circulation, no matter which of its various iterations, is abnormal in virtually every aspect of its performance. Some of these abnormalities are primarily the result of the procedure itself, and others are secondary to the fundamental disturbances of circulatory performance imposed by the ‘single’ ventricle circulation. The physiology of ventricular, systemic arterial and venopulmonary function will be described in this review. © 2006 Elsevier Ireland Ltd. All rights reserved. Keywords: palliation; Fontan circulation; Cavopulmonary anastomosis; Antriopulmonary anastomosis 1. Introduction has the profound consequences for the systemic ventricle that will discussed below. At the same time as the bidirectional In this personal overview, I will discuss the physiology of the cavopulmonary anastomosis was becoming almost uniformly Fontan circulation. Bob Freedom's contribution to our under- adopted, there was abandonment of the ‘classical’ atriopulmon- standing of complex congenital heart disease has meant that ary anastomosis, in favour of the total cavopulmonary con- many more of these patients are surviving with this physiology nection (TCPC). The ‘lateral wall’ TCPC, popularised by Marc than could have been contemplated 30 years ago. Nonetheless, deLeval [1], was shown experimentally and clinically to be they represent a very difficult group of patients and we continue haemodynamically more efficient [1,2] and set the scene for the to learn more about this unique circulation. -

A Better Approach for Left Ventricular Training In
Received: 28 September 2018 | Revised: 7 November 2018 | Accepted: 29 December 2018 DOI: 10.1111/chd.12749 ORIGINAL ARTICLE A better approach for left ventricular training in transposition of the great arteries and intact interventricular septum: Bidirectional cavopulmonary anastomosis and pulmonary artery banding Mehmet Salih Bilal MD1 | Arda Özyüksel MD1,2 | Mustafa Kemal Avşar MD1 | Şener Demiroluk MD3 | Osman Küçükosmanoğlu MD4 | Yalım Yalçın MD5 1Department of Cardiovascular Surgery, Medicana International Hospital, Abstract Istanbul, Turkey Objective: Management of the patients with transposition of the great arteries and 2 Department of Cardiovascular intact ventricular septum may be challenging beyond the newborn period. Herein, we Surgery, Biruni University, Istanbul, Turkey would like to present our alternative strategy for training the left ventricle in these 3Department of Anesthesiology, Medicana International Hospital, Istanbul, Turkey patients. 4Department of Pediatric Methods: Six patients with transposition of the great arteries and intact ventricular Cardiology, Medicana International Hospital, Istanbul, Turkey septum were evaluated in our clinic. Two of them were palliated with Glenn proce- 5Department of Pediatric dure and pulmonary banding as a definitive treatment strategy at other centers. Four Cardiology, Florence Nightingale Hospital, patients were operated on and a bidirectional cavopulmonary anastomosis in combi- Istanbul, Turkey nation with pulmonary artery banding was performed (stage‐1: palliation and ven- Correspondence tricular training) in our center. In four out of these six patients, arterial switch Arda Özyüksel, Department of Cardiovascular Surgery, Medicana operation was performed with takedown and direct re‐anastomosis of the superior International Hospital, Beylikdüzü Caddesi, vena cava to right atrium after an interstage period of 21‐30 months (stage‐2: ana- No:3, Beylikdüzü, Istanbul, Turkey. -
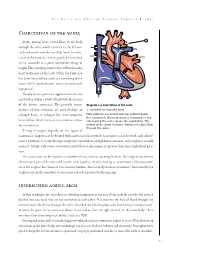
Coarctation of the Aorta Interrupted Aortic Arch
T HE P EDIATRIC C ARDIAC S URGERY I NQUEST R EPORT Coarctation of the aorta In the normal heart, blood flows to the body through the aorta, which connects to the left ven- tricle and arches over the top of the heart.In coarc- tation of the aorta,the aorta is pinched (in medical terms, coarcted) at a point somewhere along its length. This pinching restricts blood flow from the heart to the rest of the body. Often, the baby also has other heart defects, such as a narrowing of the aortic arch (in medical terms,transverse aortic arch hypoplasia). Usually no symptoms are apparent at birth, but can develop within a week of birth with the closure of the ductus arteriosus. The possible conse- Diagram 2.9 Coarctation of the aorta quences of this condition are poor feeding, an 1 – pinched or coarcted aorta enlarged heart, an enlarged liver and congestive Flow patterns are normal but are reduced below the coarctation. Blood pressure is increased in ves- heart failure. Blood pressure also increases above sels leaving the aorta above the coarctation. The the constriction. broken white arrow indicates diminished blood flow through the aorta. Timing of surgery depends on the degree of coarctation. Surgery may be delayed with mild coarctation (which sometimes is not detected until adoles- cence). However, if a baby develops congestive heart failure or high blood pressure, early surgery is usually required. A baby with severe coarctation should have early surgery to prevent long-term high blood pres- sure. The coarctation can be repaired in a number of ways without opening the heart.The surgeon can remove the narrowed part of the aorta and sew the ends together, thereby creating an anastomosis. -

Congenital Heart Disease
GUEST EDITORIAL Congenital heart disease Pediatric Anesthesia is the only anesthesia journal ded- who developed hypoglycemia were infants. (9). Steven icated exclusively to perioperative issues in children and Nicolson take the opposite approach of ‘first do undergoing procedures under anesthesia and sedation. no harm’ (10). If we do not want ‘tight glycemic con- It is a privilege to be the guest editor of this special trol’ because of concern about hypoglycemic brain issue dedicated to the care of children with heart dis- injury, when should we start treating blood sugars? ease. The target audience is anesthetists who care for There are no clear answers based on neurological out- children with heart disease both during cardiac and comes in children. non-cardiac procedures. The latter takes on increasing Williams and Cohen (11) discuss the care of low importance as children with heart disease undergoing birth weight (LBW) infants and their outcomes. Pre- non-cardiac procedures appear to be at a higher risk maturity and LBW are independent risk factors for for cardiac arrest under anesthesia than those without adverse outcomes after cardiac surgery. Do the anes- heart disease (1). We hope the articles in this special thetics we use add to this insult? If prolonged exposure issue will provide guidelines for management and to volatile anesthetics is bad for the developing neona- spark discussions leading to the production of new tal brain, would avoiding them make for improved guidelines. outcomes? Wise-Faberowski and Loepke (12) review Over a decade ago Austin et al. (2) demonstrated the current research in search of a clear answer and the benefits of neurological monitoring during heart conclude that there isn’t one. -

Pediatric Radiology
2013 RSNA (Filtered Schedule) Sunday, December 01, 2013 10:30-12:00 PM • VSPD11 • Room: S100AB • Pediatric Radiology Series: Pediatric Neuroimaging I 10:45-12:15 PM • SPOI11 • Room: E353C • Oncodiagnosis Panel: Pediatric Sarcoma (An Interactive Session) 12:30-01:00 PM • CL-PDS-SUA • Room: S101AB • Pediatric Radiology - Sunday Posters and Exhibits (12:30pm - 1:00pm) 01:00-01:30 PM • CL-PDS-SUB • Room: S101AB • Pediatric Radiology - Sunday Posters and Exhibits (1:00pm - 1:30pm) 02:00-03:30 PM • VSPD12 • Room: S102AB • Pediatric Radiology Series: Pediatric Musculoskeletal Monday, December 02, 2013 08:30-10:00 AM • RC224 • Room: E353B • Mentored Case Approach to Pediatric Cardiovascular Disease 1: Vascular Disease (An Interactive Session) 08:30-12:00 PM • VSPD21 • Room: S102AB • Pediatric Radiology Series: Fetal - Neonatal Imaging 12:15-12:45 PM • CL-PDS-MOA • Room: S101AB • Pediatric Radiology - Monday Posters and Exhibits (12:15pm - 12:45pm) 12:45-01:15 PM • CL-PDS-MOB • Room: S101AB • Pediatric Radiology - Monday Posters and Exhibits (12:45pm - 1:15pm) 03:00-04:00 PM • SSE21 • Room: S102AB • Pediatric (Neuroimaging) Tuesday, December 03, 2013 08:30-10:00 AM • RC324 • Room: S402AB • Mentored Case Approach to Pediatric Cardiovascular Disease 2: Cardiac Disease (An Interactive Session) 08:30-12:00 PM • VSPD31 • Room: S102AB • Pediatric Radiology Series: Chest/Cardiovascular Imaging I 12:15-12:45 PM • CL-PDS-TUA • Room: S101AB • Pediatric Radiology - Tuesday Scientific Posters and Exhibits (12:15pm - 12:45pm) 12:45-01:15 PM • CL-PDS-TUB • -

An Overview of Mechanical Circulatory Support in Single-Ventricle Patients
161 Review Article An overview of mechanical circulatory support in single-ventricle patients Jacob R. Miller1, Timothy S. Lancaster1, Connor Callahan2, Aaron M. Abarbanell3, Pirooz Eghtesady3 1Division of Cardiothoracic Surgery, 2Department of Surgery, Barnes-Jewish Hospital/Washington University School of Medicine, St. Louis, MO, USA; 3Section of Pediatric Cardiothoracic Surgery, St. Louis Children’s Hospital/Washington University School of Medicine, St. Louis, MO, USA Contributions: (I) Conception and design: JR Miller, AM Abarbanell, P Eghtesady; (II) Administrative support: AM Abarbanell, P Eghtesady; (III) Provision of study materials or patients: None; (IV) Collection and assembly of data: JR Miller, TS Lancaster, C Callahan; (V) Data analysis and interpretation: JR Miller, AM Abarbanell, P Eghtesady; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Pirooz Eghtesady, MD, PhD. Chief of Pediatric Cardiothoracic Surgery, St. Louis Children’s Hospital, One Children’s Place, Suite 5 South, St. Louis, MO 63110, USA. Email: [email protected]. Abstract: The population of people with a single-ventricle is continually increasing due to improvements across the spectrum of medical care. Unfortunately, a proportion of these patients will develop heart failure. Often, for these patients, mechanical circulatory support (MCS) represents the only available treatment option. While single-ventricle patients currently represent a small proportion of the total number of patients who receive MCS, as the single-ventricle patient population increases, this number will increase as well. Outcomes for these complex single-ventricle patients who require MCS has begun to be evaluated. When considering the entire population, survival to hospital discharge is 30–50%, though this must be considered with the significant heterogeneity of the single-ventricle patient population. -

Pulmonary-Atresia-Mapcas-Pavsdmapcas.Pdf
Normal Heart © 2012 The Children’s Heart Clinic NOTES: Children’s Heart Clinic, P.A., 2530 Chicago Avenue S, Ste 500, Minneapolis, MN 55404 West Metro: 612-813-8800 * East Metro: 651-220-8800 * Toll Free: 1-800-938-0301 * Fax: 612-813-8825 Children’s Minnesota, 2525 Chicago Avenue S, Minneapolis, MN 55404 West Metro: 612-813-6000 * East Metro: 651-220-6000 © 2012 The Children’s Heart Clinic Reviewed March 2019 Pulmonary Atresia, Ventricular Septal Defect and Major Aortopulmonary Collateral Arteries (PA/VSD/MAPCAs) Pulmonary atresia (PA), ventricular septal defect (VSD) and major aortopulmonary collateral arteries (MAPCAs) is a rare type of congenital heart defect, also referred to as Tetralogy of Fallot with PA/MAPCAs. Tetralogy of Fallot (TOF) is the most common cyanotic heart defect and occurs in 5-10% of all children with congenital heart disease. The classic description of TOF includes four cardiac abnormalities: overriding aorta, right ventricular hypertrophy (RVH), large perimembranous ventricular septal defect (VSD), and right ventricular outflow tract obstruction (RVOTO). About 20% of patients with TOF have PA at the infundibular or valvar level, which results in severe right ventricular outflow tract obstruction. PA means that the pulmonary valve is closed and not developed. When PA occurs, blood can not flow through the pulmonary arteries to the lungs. Instead, the child is dependent on a patent ductus arteriosus (PDA) or multiple systemic collateral vessels (MAPCAs) to deliver blood to the lungs for oxygenation. These MAPCAs usually arise from the de- scending aorta and subclavian arteries. Commonly, the pulmonary arteries are abnormal, with hypoplastic (small and underdeveloped) central and branch pulmonary arteries and/ or non-confluent central pulmonary arteries. -

Prenatal Diagnosis, Associated Findings and Postnatal Outcome Of
J. Perinat. Med. 2019; 47(3): 354–364 Ingo Gottschalka,*, Judith S. Abela, Tina Menzel, Ulrike Herberg, Johannes Breuer, Ulrich Gembruch, Annegret Geipel, Konrad Brockmeier, Christoph Berg and Brigitte Strizek Prenatal diagnosis, associated findings and postnatal outcome of fetuses with double outlet right ventricle (DORV) in a single center https://doi.org/10.1515/jpm-2018-0316 anomalies, 30 (66.7%) had extracardiac anomalies and 13 Received September 18, 2018; accepted November 26, 2018; (28.9%) had chromosomal or syndromal anomalies. Due previously published online December 20, 2018 to their complex additional anomalies, five (11.1%) of our Abstract 45 fetuses had multiple malformations and were highly suspicious for non-chromosomal genetic syndromes, Objective: To assess the spectrum of associated anoma- although molecular diagnosis could not be provided. Dis- lies, the intrauterine course, postnatal outcome and orders of laterality occurred in 10 (22.2%) fetuses. There management of fetuses with double outlet right ventricle were 17 terminations of pregnancy (37.8%), two (4.4%) (DORV). intrauterine and seven (15.6%) postnatal deaths. Nineteen Methods: All cases of DORV diagnosed prenatally over a of 22 (86.4%) live-born children with an intention to treat period of 8 years were retrospectively collected in a single were alive at last follow-up. The mean follow-up among tertiary referral center. All additional prenatal findings survivors was 32 months (range, 2–72). Of 21 children who were assessed and correlated with the outcome. The accu- had already undergone postnatal surgery, eight (38.1%) racy of prenatal diagnosis was assessed. achieved biventricular repair and 13 (61.9%) received Results: Forty-six cases of DORV were diagnosed pre- univentricular palliation. -

Glenn Surgery: a Safe Procedure in the Path of Univentricular Correction
Boletín Médico del Hospital Infantil de México RESEARCH ARTICLE Glenn surgery: a safe procedure in the path of univentricular correction Guadalupe Hernández-Morales1*, Alejandro Bolio-Cerdán2, Sergio Ruiz-González2, Patricia Romero-Cárdenas2, and Miguel A. Villasís-Keever3 1Departamento de Cirugía Cardiaca en Pediatría, Hospital Militar de Especialidades de la Mujer y Neonatología; 2Departamento de Cirugía Cardiovascular, Hospital Infantil de México Federico Gómez; 3Unidad de Investigación en Epidemiología Clínica, Unidad Médica de Alta Especialidad, Hospital de Pediatría, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social. Mexico City, Mexico Abstract Background: This study describes 35 years of experience in a tertiary care level hospital that treats cardiac patients with univentricular heart physiology who underwent Glenn surgery. Methods: The study consisted of a retrospective analysis of patients who underwent Glenn surgery, including variables related to pre-operative, intra-operative, and post-operative mor- bidity and mortality. Results: From 1980 to 2015, 204 Glenn surgeries were performed. The most common heart disease was tricuspid atresia IB (19.2%). In 48.1% of the cases, the procedure was performed with antegrade flow. A bilateral Glenn procedure was performed in 12.5% of the cases and 10.3% were carried out without using a cardiopulmonary bypass pump. Reported complications included infections, bleeding, arrhythmias, chylothorax, neurological alterations, and pleural effusion. The mortality rate was 2.9%. Conclusions: Glenn surgery is a palliative surgery with good results. It significantly improves patient quality of life over a long period until a total cavopulmonary shunt is performed. The complications observed are few, and the mortality rate is low. -

Outcome of the Norwood Operation in Patients with Hypoplastic Left Heart Syndrome: a 12-Year Single-Center Survey
Furck et al Congenital Heart Disease Outcome of the Norwood operation in patients with hypoplastic left heart syndrome: A 12-year single-center survey Anke Katharina Furck, MD,a Anselm Uebing, MD,a Jan Hinnerk Hansen,a Jens Scheewe, MD,b Olaf Jung, MD,a Gunther Fischer, MD,a Carsten Rickers, MD,a Tim Holland-Letz, MSc,c and Hans-Heiner Kramer, MDa Objective: Recent advances in perioperative care have led to a decrease in mortality of children with hypoplastic left heart syndrome undergoing the Norwood operation. This study aimed to evaluate the outcome of the Nor- wood operation in a single center over 12 years and to identify clinical and anatomic risk factors for adverse early CHD and longer term outcome. Methods: Full data on all 157 patients treated between 1996 and 2007 were analyzed. Results: Thirty-day mortality of the Norwood operation decreased from 21% in the first 3 years to 2.5% in the last 3 years. The estimated exponentially weighted moving average of early mortality after 157 Norwood oper- ations was 2.3%. Risk factors were an aberrant right subclavian artery, the use and duration of circulatory arrest, and the duration of total support time. The anatomic subgroup mitral stenosis/aortic atresia and female gender tended to show an increased early mortality. In the group of patients who required postoperative cardiopulmonary resuscitation, the ascending aorta was significantly smaller than in the remainder (3.03 Æ 1.05 vs 3.63 Æ 1.41 mm). Interstage mortality was 15% until the initiation of a home surveillance program in 2005, which has zeroed it so far.