Control of Sulfidity in a Modern Kraft Pulp Mill
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Entrained-Flow Gasification of Black Liquor and Pyrolysis Oil
LICENTIATE T H E SIS Department of Engineering Sciences and Mathematics Division of Energy Science Yawer Jafri Entrained-Flow Gasification of Black Liquor and Pyrolysis Gasification of Black Liquor and Pyrolysis Jafri Entrained-Flow Yawer ISSN 1402-1757 Entrained-Flow Gasification of ISBN 978-91-7583-761-1 (print) ISBN 978-91-7583-762-8 (pdf) Black Liquor and Pyrolysis Oil Luleå University of Technology 2016 Pilot-Scale and Equilibrium Modelling Studies of Catalytic Co-gasification Yawer Jafri Energy Engineering Licentiate Thesis Entrained-Flow Gasification of Black Liquor and Pyrolysis Oil Pilot-scale and Equilibrium Modelling Studies of Catalytic Co-gasification Yawer Jafri Energy Engineering Division of Energy Science Department of Environmental Sciences and Mathematics Luleå University of Technology a Printed by Luleå University of Technology, Graphic Production 2016 ISSN 1402-1757 ISBN 978-91-7583-761-1 (print) ISBN 978-91-7583-762-8 (pdf) Luleå 2016 www.ltu.se b Abstract The last couple of decades have seen entrained-flow gasification of black liquor (BL) undergo an incremental process of technical development as an alternative to combustion in a recovery boiler. The ability of the technology to combine chemical recovery with the production of clean syngas renders it a promising candidate for the transformation of chemical pulp mills into integrated forest biorefineries. However, techno-economic assessments have shown that blending BL with the more easily transportable pyrolysis oil (PO) can not only increase the energy efficiency of the conversion process for methanol production, but also remove a significant roadblock to commercial deployment by partially decoupling production capacity from BL availability. -

Mountain Pine Beetle-Attacked Lodgepole Pine for Pulp and Papermaking
Operational extractives management from- mountain pine beetle-attacked lodgepole pine for pulp and papermaking Larry Allen and Vic Uloth Mountain Pine Beetle Working Paper 2007-15 Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre, 506 West Burnside Road, Victoria, BC V8Z 1M5 (250) 363-0600 • cfs.nrcan.gc.ca/regions/pfc Natural Resources Ressources naturelles Canada Canada Canadian Forest Service canadien Service des forêts Operational extractives management from mountain pine beetle-attacked lodgepole pine for pulp and papermaking Larry Allen and Vic Uloth Mountain Pine Beetle Initiative W orking Paper 2007œ15 Paprican 3800 W esbrook Mall Vancouver, B.C. V6S 2L9 Mountain Pine Beetle Initiative PO # 8.43 Natural Resources Canada Canadian Forest Service Pacific Forestry Centre 506 W est Burnside Road Victoria, British Columbia V8Z 1M5 Canada 2007 ≤ Her Majesty the Queen in Right of Canada 2007 Printed in Canada Library and Archives Canada Cataloguing in Publication Allen, Larry Operational extractives m anagem ent from m ountain pine beetle-attached lodgepole pine from pulp and paperm aking / Larry Allen and Vic Uloth. (Mountain Pine Beetle Initiative working paper 2007-15) "Mountain Pine Beetle Initiative, Canadian Forest Service". "MPBI Project # 8.43". "Paprican". Includes bibliographical references: p. Includes abstract in French. ISBN 978-0-662-46480-8 Cat. no.: Fo143-3/2007-15E 1. Pulping--British Colum bia--Quality control. 2. Pulping--Alberta--Quality control. 3. Paper m ills-- Econom ic aspects--British Colum bia. 4. Pulp m ills--Econom ic aspects--Alberta. 5. Lodgepole pine--Diseases and pests–Econom ic aspects. 6. Mountain pine beetle--Econom ic aspects. -

Basics of Kraft Pulping
Lignin Wood is composed of many chemical components, primarily extractives, carbohydrates, and lignin, which are distributed nonuniformly as the result of anatomical structure. Lignin is derived from the Latin term lignum, which means wood.1 Anselme Payen (1838) was the first to recognize the composite nature of wood and referred to a carbon- rich substance as the “encrusting material” which embedded cellulose in the wood. Schulze (1865) later defined this encrusting material as lignin. Lignin has been described as a random, three-dimensional network polymer comprised of variously linked phenylpropane units.2 Lignin is the second most abundant biological material on the planet, exceeded only by cellulose and hemicellulose, and comprises 15-25% of the dry weight of woody plants. This macromolecule plays a vital role in providing mechanical support to bind plant fibers together. Lignin also decreases the permeation of water through the cell walls of the xylem, thereby playing an intricate role in the transport of water and nutrients. Finally, lignin plays an important function in a plant’s natural defense against degradation by impeding penetration of destructive enzymes through the cell wall. Although lignin is necessary to trees, it is undesirable in most chemical papermaking fibers and is removed by pulping and bleaching processes. 1.1.1 Biosynthesis Plant lignins can be broadly divided into three classes: softwood (gymnosperm), hardwood (angiosperm) and grass or annual plant (graminaceous) lignin.3 Three different phenylpropane units, or monolignols, are responsible for lignin biosynthesis.4 Guaiacyl lignin is composed principally of coniferyl alcohol units, while guaiacyl-syringyl lignin contains monomeric units from coniferyl and sinapyl alcohol. -

Corrugated Board Structure: a Review M.C
ISSN: 2395-3594 IJAET International Journal of Application of Engineering and Technology Vol-2 No.-3 Corrugated Board Structure: A Review M.C. Kaushal1, V.K.Sirohiya2 and R.K.Rathore3 1 2 Assistant Prof. Mechanical Engineering Department, Gwalior Institute of Information Technology,Gwalior, Assistant Prof. Mechanical Engineering 3 Departments, Gwalior Engineering College, Gwalior, M. Tech students Maharanapratap College of Technology, Gwalior, [email protected] [email protected] [email protected] ABSTRACT Corrugated board is widely used in the packing industry. The main advantages are lightness, recyclability and low cost. This makes the material the best choice to produce containers devoted to the shipping of goods. Furthermore examples of structure design based on corrugated boards can be found in different fields. Structural analysis of paperboard components is a crucial topic in the design of containers. It is required to investigate their strength properties because they have to protect the goods contained from lateral crushing and compression loads due to stacking. However in this paper complete and detailed information are presented. Keywords: - corrugated boards, recyclability, compression loads. Smaller flutes offer printability advantages as well as I. INTRODUCTION structural advantages for retail packaging. Corrugated board is essentially a paper sandwich consisting of corrugated medium layered between inside II. HISTORY and outside linerboard. On the production side, corrugated In 1856 the first known corrugated material was patented is a sub-category of the paperboard industry, which is a for sweatband lining in top hats. During the following four sub-category of the paper industry, which is a sub-category decades other forms of corrugated material were used as of the forest products industry. -
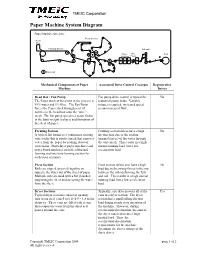
Paper Machine System Diagram
TMEIC Corporation Paper Machine System Diagram Paper Machine One-Line Press Section Head Box Forming Section Calender Dryer Size Reel Sections Press Driven roll Mechanical Components of Paper Associated Drive Control Concepts Regenerative Machine Drives Head Box / Fan Pump Fan pump drive control is typical for No The Paper stock at this point in the process is industrial pump loads. Variable 99% water and 1% fiber. The Fan Pump torque is required, increased speed forces the Paper stock through a set of means increased flow. nozzles in the head box onto the “wire” mesh. The fan pump speed is a major factor in the basis weight (caliper) and formation of the sheet of paper. Forming Section Forming section drives have a high No A typical flat former is a continuous rotating friction load due to the suction wire (today this is plastic) mesh that removes (normal forces) of the water through water from the paper by sucking it out of the wire mesh. This results in a high suspension. Multi-layer paper machines and normal running load, but a low paper board machines include additional acceleration load. forming sections (one forming section for each layer of paper). Press Section Press section drives also have a high No Rolls are nipped (pressed) together to load due to the strong forces in the nip squeeze the water out of the sheet of paper. between the rolls deforming the felts Multiple rolls are used with a felt (blanket) and roll. This results in a high normal supporting the sheet and accepting the water running load, but a low acceleration from the sheet. -

TECHNICAL REPORT – PATENT ANALYSIS Enhancing Productivity in the Indian Paper and Pulp Sector
TECHNICAL REPORT – PATENT ANALYSIS Enhancing Productivity in the Indian Paper and Pulp Sector 2018 TABLE OF contEnts ACKNOWLEDGEMENTS 10 EXECUTIVE SUMMARY 11 1 INTRODUCTION 13 2 OVERVIEW OF THE PULP AND PAPER SECTOR 15 2.1. Status of the Indian Paper Industry 15 2.2. Overview of the Pulp and Papermaking Process 20 2.3. Patenting in the Paper and Pulp Industry: A Historical Perspective 22 2.4. Environmental Impact of the Pulp and Paper Industry 25 3 METHODOLOGY 27 3.1. Search Strategy 27 4 ANALYSIS OF PATENT DOCUMENTS USING GPI 31 4.1. Papermaking; Production of Cellulose (IPC or CPC class D21) 31 4.2. Analysis of Patenting Activity in Different Technology Areas using GPI 38 5 ANALYSIS OF THE INDIAN PATENT SCENARIO WITHIN THE CONTEXT OF THIS REPORT 81 5.1. Analysis of Patents Filed in India 81 6 CONCLUDING REMARKS 91 REFERENCES 93 ANNEXURE 94 Annexure 1. Technologies related to paper manufacturing 94 Annexure 2. Sustainable/green technologies related to pulp and paper sector 119 Annexure 3. Emerging Technology Areas 127 List OF FIGURES Figure 2.1: Geographical Spread of Figure 4.11: (d) Applicant vs. Date of Indian Paper Mills .................................16 Priority Graph: Paper-Making Machines Figure 2.2: Share of Different Segments and Methods ........................................42 in Total Paper Production .......................19 Figure 4.11: (e) Applicant vs. Date of Figure 2.3: Variety Wise Production of Priority Graph: Calendars and Accessories ..43 Paper from Different Raw Materials ........19 Figure 4.11: (f) Applicant vs. Date of Figure 2.4: Different Varieties of Paper Priority Graph: Pulp or Paper Comprising Made from Various Raw Materials ..........19 Synthetic Cellulose or Non-Cellulose Fibres ..43 Figure 2.5: Diagram of a Process Block Figure 4.11: (g) Applicant vs. -

American Ft Forest & Paper 2QE5
A 1 , i American Ft Forest & Paper 2QE5; Association , - May 6, 2015 Via Hand Delivery and by Email to iones.iim(a^epa.gov Administrator Gina McCarthy (1101A) Office of the Administrator Environmental Protection Agency 1200 Pennsylvania Avenue, N.W. Washington, DC 20460 Re: Reguest for Full Exemption of Four Pulping Chemicals from the TSCA Chemical Data Reporting Rule Reguirements Dear Administrator McCarthy: The American Forest & Paper Association (AF&PA) hereby petitions EPA to amend the Chemical Data Reporting rule (CDR), 40 C.F.R. Part 711, to exempt from all CDR requirements four pulping chemicals involved in the manufacture of paper and other pulp-based products. The four pulping chemicals are complex mixtures used in the kraft pulping process: • Sulfite Liquors and Cooking Liquors, white (CAS No. 68131-33-9) (white liquor) • Sulfite Liquors and Cooking Liquors, spent (CAS No. 66071-92-9) (black liquor) • Sulfite Liquors and Cooking Liquors, spent, oxidized (CAS No. 68514-09-0) (black liquor, oxidized) • Sulfite Liquors and Cooking Liquors, green (CAS No. 68131-30-6) (green liquor) Each of these substances is manufactured and recycled onsite in a continuous closed loop. EPA has an enormous amount of information about these pulping chemicals and little current interest in them, as their potential risks are well understood and adequately managed. 1101 K Street, N.W., Suite 700 • Was hington, D.C.20005 • (202) 463•2700 •afandpa.org May 6, 2015 Page 2 This petition first identifies the four pulping chemicals in greater detail and explains why the kraft chemical regeneration process has led to inflated production volumes for these pulping chemicals in CDR data. -
Making Paper from Trees
Making Paper from Trees Forest Service U.S. Department of Agriculture FS-2 MAKING PAPER FROM TREES Paper has been a key factor in the progress of civilization, especially during the past 100 years. Paper is indispensable in our daily life for many purposes. It conveys a fantastic variety and volume of messages and information of all kinds via its use in printing and writing-personal and business letters, newspapers, pamphlets, posters, magazines, mail order catalogs, telephone directories, comic books, school books, novels, etc. It is difficult to imagine the modern world without paper. Paper is used to wrap packages. It is also used to make containers for shipping goods ranging from food and drugs to clothing and machinery. We use it as wrappers or containers for milk, ice cream, bread, butter, meat, fruits, cereals, vegetables, potato chips, and candy; to carry our food and department store purchases home in; for paper towels, cellophane, paper handkerchiefs and sanitary tissues; for our notebooks, coloring books, blotting paper, memo pads, holiday greeting and other “special occasion’’ cards, playing cards, library index cards; for the toy hats, crepe paper decorations, paper napkins, paper cups, plates, spoons, and forks for our parties. Paper is used in building our homes and schools-in the form of roofing paper, and as paperboard- heavy, compressed product made from wood pulp-which is used for walls and partitions, and in such products as furniture. Paper is also used in linerboard, “cardboard,” and similar containers. Wood pulp is the principal fibrous raw material from which paper is made, and over half of the wood cut in this country winds up in some form of paper products. -

A Dynamic Na/S Balance of a Kraft Pulp Mill
A dynamic Na/S balance of a kraft pulp mill Modeling and simulation of a kraft pulp mill using WinGEMS En dynamisk Na/S balans av ett sulfatbruk Modellering och simulering av ett sulfatbruk i WinGEMS Per Andersson Faculty of Health, Science and Technology Department of Engineering and Chemical Science, Chemical Engineering, Karlstad University Master theisis, 30 credits Supervisors: Niklas Kvarnström (KAU), Maria Björk (Stora Enso), Rickard Wadsborn (Stora Enso) Examinat or: Lars Järnström (KAU) 2014 -01-08 Version : 2.0 Abstract The main scope of this thesis was to create a simulation model of a kraft pulp mill and produce a dynamic Na/S balance. The model was made in WinGEMS 5.3 and the method consisted of implementing a static Na/S balance from the mill and created a model that described this chemical balance. Input data from the mill was collected and implemented in the model. A number of different cases were simulated to predict the effects of different process changes over time, dynamic balances. The result from the static balance showed that the model can describes the mill case. The result from the dynamic simulation showed that the model can be used to predict the effect of process changes over shorter periods of time. Executive Summary In the kraft mill the chemical balance is of interest to minimize the production cost. Normally there is an excess of sulfur and low levels of sodium, compared to what the process requires. In the future, the pulp mill will most likely produce other products than just pulp. These new production processes will also most likely affect the sodium and sulfur balance and there is a need to be able to predict this change. -

Make Your Own Paper Students Investigate the Papermaking Process by Trying It Themselves
Make Your Own Paper Students investigate the papermaking process by trying it themselves. Students are Activity thrilled to find that they can make paper and that their product is practical, as well as beautiful. See the PLT website, www.plt.org, to watch a video of the paper-making 51 process used in this activity. Levels OBJECTIVE ASSESSMENT OPPORTUNITIES Grades 1-8 Subjects n Students will describe the steps of the paper- n Ask younger students to write the directions Science, Social Studies, making process and identify the elements and for making paper on the piece of recycled paper Language Arts, Visual Arts outputs of the process. that they made. n Concepts Have students use concept mapping, graphics software, or write a script for a n By reducing waste and recy- video that explains the papermaking process. cling materials, individuals and societies can extend the value and utility of resources and can promote environ- mental quality. (2.7) The process begins when trees, grown BACKGROUND Skills especially for papermaking, are harvested Observing, Organizing and transported to a paper mill. At the Information, Comparing and Paper is a simple material. It is essentially mill, large machines strip away bark and Contrasting a mat held together by a fiber’s rough- shred the logs into millions of chips the ness, and can be made from almost any size of breakfast cereal. The wood chips fibrous material such as cotton, hemp, travel on conveyors to gigantic “pulp Higher Order Thinking, Paired/ flax, wood or recycled paper. And yet, this Cooperative Learning, Realia/ cookers,” where chemicals and steam are Hands-on Learning simple product has a tremendous effect added. -

Paper Recycling Technology Detailed Part 1A
Paper Recycling Technology and Science Dr. Richard A. Venditti Paper Science and Engineering Forest Biomaterials Department North Carolina State University Lecture: Paper recycling and technology course introduction and objectives Dr. Richard Venditti Faculty member in the Paper Science and Engineering Program in the Forest Biomaterials Department at North Carolina State University PhD in Chemical Engineering, BS in Pulp and Paper Science and Chemical Engineering Research areas: � Paper recycling � Utilization of forest/agricultural materials for new applications � Life cycle analysis Named a TAPPI Fellow in 2012 Relevant research projects: – The detection of adhesive contaminants – The changes in fibers upon recycling – Automatic sorting of recovered papers – Flotation deinking surfactants – Agglomeration deinking – Screening phenomena and pressure sensitive adhesives – Deposition of adhesive contaminants – Neural networks to control deinking operations – Sludge conversion to bio-ethanol and to bio- materials Course Outline The US Paper Recycling Industry Recovered Paper Grades and Contaminants Effect of Recycling on Fibers/Paper Unit Operations � Pulping, Cleaning, Screening, Washing, Flotation, Dispersion, Bleaching, ….. Image Analysis, Deinking Chemicals System Design Advanced/Additional Topics Course Activities Viewing of the Videos of Lectures � Base lectures by Venditti � Guest lectures from industry leaders Homework assignments Final Exam Critical Issues in Recycling: Going deeper into the recovered paper stream -

Odorless Pulp Mill in Operation
Published May 26, 2017 Odorless Pulp Mill Odorless pulp mill in successful operation Executive Summary The sulphur in the cooking process can result in bad odor in the surroundings of a Kraft pulp mill. Together with the odor, sulphur compounds can also be an environmental problem since they are released to the atmosphere. Although emissions are becoming lower as mills are upgraded, odors have continued to be a problem for people living close to the mills, because of the very low odor threshold of sulphur compounds. Valmet has delivered a unique, practically odorless mill to CMPC Riograndese Ltda in Guaíba, Brazil. The design guidelines were to not vent odorous gases and to aim for zero smells. The gas handling system collects and controls odors from more than 100 sources and all process areas are included. Incineration of non-condensable gases is ensured through multiple, simultaneously available incineration locations. According to CMPC it can be said that the system availability is practically 100% and there is always a system ready to handle NCG and prevent gases from being emitted to the atmosphere. This paper describes the emphasis CMPC placed in the concept of the odorless mill in Guaíba and its surroundings and outlines the chosen technical solutions to achieve this target. The paper emphasizes the necessity of ensuring that odorous gases are treated also in shutdown and emergency situations, and explains how this has been achieved at the mill. © Valmet Page | 1 Published May 26, 2017 Odorless Pulp Mill Background No venting of odorous gases and a "zero smell" pulp mill.