A Revision of Calyptochloa CE Hubb.(Poaceae), with Two New
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Grass Genera in Townsville
Grass Genera in Townsville Nanette B. Hooker Photographs by Chris Gardiner SCHOOL OF MARINE and TROPICAL BIOLOGY JAMES COOK UNIVERSITY TOWNSVILLE QUEENSLAND James Cook University 2012 GRASSES OF THE TOWNSVILLE AREA Welcome to the grasses of the Townsville area. The genera covered in this treatment are those found in the lowland areas around Townsville as far north as Bluewater, south to Alligator Creek and west to the base of Hervey’s Range. Most of these genera will also be found in neighbouring areas although some genera not included may occur in specific habitats. The aim of this book is to provide a description of the grass genera as well as a list of species. The grasses belong to a very widespread and large family called the Poaceae. The original family name Gramineae is used in some publications, in Australia the preferred family name is Poaceae. It is one of the largest flowering plant families of the world, comprising more than 700 genera, and more than 10,000 species. In Australia there are over 1300 species including non-native grasses. In the Townsville area there are more than 220 grass species. The grasses have highly modified flowers arranged in a variety of ways. Because they are highly modified and specialized, there are also many new terms used to describe the various features. Hence there is a lot of terminology that chiefly applies to grasses, but some terms are used also in the sedge family. The basic unit of the grass inflorescence (The flowering part) is the spikelet. The spikelet consists of 1-2 basal glumes (bracts at the base) that subtend 1-many florets or flowers. -

By H.D.V. PRENDERGAST a Thesis Submitted for the Degree of Doctor of Philosophy of the Australian National University. January 1
STRUCTURAL, BIOCHEMICAL AND GEOGRAPHICAL RELATIONSHIPS IN AUSTRALIAN c4 GRASSES (POACEAE) • by H.D.V. PRENDERGAST A thesis submitted for the degree of Doctor of Philosophy of the Australian National University. January 1987. Canberra, Australia. i STATEMENT This thesis describes my own work which included collaboration with Dr N .. E. Stone (Taxonomy Unit, R .. S .. B.S .. ), whose expertise in enzyme assays enabled me to obtain comparative information on enzyme activities reported in Chapters 3, 5 and 7; and with Mr M.. Lazarides (Australian National Herbarium, c .. s .. r .. R .. O .. ), whose as yet unpublished taxonomic views on Eragrostis form the basis of some of the discussion in Chapter 3. ii This thesis describes the results of research work carried out in the Taxonomy Unit, Research School of Biological Sciences, The Australian National University during the tenure of an A.N.U. Postgraduate Scholarship. iii ACKNOWLEDGEMENTS My time in the Taxonomy Unit has been a happy one: I could not have asked for better supervision for my project or for a more congenial atmosphere in which to work. To Dr. Paul Hattersley, for his help, advice, encouragement and friendship, I owe a lot more than can be said in just a few words: but, Paul, thanks very much! To Mr. Les Watson I owe as much for his own support and guidance, and for many discussions on things often psittacaceous as well as graminaceous! Dr. Nancy Stone was a kind teacher in many days of enzyme assays and Chris Frylink a great help and friend both in and out of the lab •• Further thanks go to Mike Lazarides (Australian National Herbarium, c.s.I.R.O.) for identifying many grass specimens and for unpublished data on infrageneric groups in Eragrostis; Dr. -

The Vegetation of Granitic Outcrop Communities on the New England Batholith of Eastern Australia
547 The vegetation of granitic outcrop communities on the New England Batholith of eastern Australia John T. Hunter and Peter J. Clarke Hunter, John T. and Clarke, Peter J. (Division of Botany, University of New England, Armidale, NSW 2350) 1998. The vegetation of granitic outcrop communities on the New England Batholith of eastern Australia. Cunninghamia 5 (3): 547–618. The vegetation of 22 areas of granitic outcrops on the New England Batholith has been surveyed using semi-quantitative quadrat sampling. In total 399 0.1 ha quadrats were placed on 216 outcrops. Twenty-eight plant communities in nine major groups and an additional unsurveyed community are circumscribed. A high number of nationally rare or threatened taxa, many of which are restricted to outcrop areas, have been found in these communities along with many taxa of special note. Previous studies have over-emphasised structure which can vary considerably with negligible floristic change. Suggestions are made on potential areas for reservation. Introduction Studies concentrating on the vegetation of granitic outcrops have been undertaken throughout the world (e.g. Whitehouse 1933; Oosting & Anderson 1937; McVaugh 1943; Keever et al. 1951; Keever 1957; Hambler 1964; Murdy et al. 1970; Sharitz & McCormick 1973; Rundel 1975; Shure & Fagsdale 1977; Wyatt 1977; Phillips 1981; Phillips 1982; Wyatt 1981; Baskin & Baskin 1982; Walters 1982; Burbanck & Phillips 1983; Wyatt 1984a, b; Uno & Collins 1987; Baskin & Baskin 1988; Houle & Phillips 1988; Houle & Phillips 1989a, b; Houle 1990; Porembski et al. 1994; Ibisch et al. 1995; Porembski 1995; Porembski et al. 1996). Research into outcrops, and in particular granitic outcrops, has culminated in the formation of the ‘Inselberg-Projeckt’ supported by the Deutsche Forschungsge-meinschaft (Porembski et al. -

Lose the Plot: Cost-Effective Survey of the Peak Range, Central Queensland
Lose the plot: cost-effective survey of the Peak Range, central Queensland. Don W. Butlera and Rod J. Fensham Queensland Herbarium, Environmental Protection Agency, Mt Coot-tha Botanic Gardens, Mt Coot-tha Road, Toowong, QLD, 4066 AUSTRALIA. aCorresponding author, email: [email protected] Abstract: The Peak Range (22˚ 28’ S; 147˚ 53’ E) is an archipelago of rocky peaks set in grassy basalt rolling-plains, east of Clermont in central Queensland. This report describes the flora and vegetation based on surveys of 26 peaks. The survey recorded all plant species encountered on traverses of distinct habitat zones, which included the ‘matrix’ adjacent to each peak. The method involved effort comparable to a general flora survey but provided sufficient information to also describe floristic association among peaks, broad habitat types, and contrast vegetation on the peaks with the surrounding landscape matrix. The flora of the Peak Range includes at least 507 native vascular plant species, representing 84 plant families. Exotic species are relatively few, with 36 species recorded, but can be quite prominent in some situations. The most abundant exotic plants are the grass Melinis repens and the forb Bidens bipinnata. Plant distribution patterns among peaks suggest three primary groups related to position within the range and geology. The Peak Range makes a substantial contribution to the botanical diversity of its region and harbours several endemic plants among a flora clearly distinct from that of the surrounding terrain. The distinctiveness of the range’s flora is due to two habitat components: dry rainforest patches reliant upon fire protection afforded by cliffs and scree, and; rocky summits and hillsides supporting xeric shrublands. -

C4 Photosynthetic Evolution
C4 PHOTOSYNTHETIC EVOLUTION: SUB-TYPES, DIVERSITY, AND FUNCTION WITHIN THE GRASS TRIBE PANICEAE _______________________________________ A Dissertation presented to the Faculty of the Graduate School at the University of Missouri-Columbia _______________________________________________________ In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy _____________________________________________________ by JACOB DANIEL WASHBURN Dr. J. Chris Pires, Dissertation Supervisor MAY 2017 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled: C4 PHOTOSYNTHETIC EVOLUTION: SUB-TYPES, DIVERSITY, AND FUNCTION WITHIN THE GRASS TRIBE PANICEAE Presented by Jacob Daniel Washburn, a candidate for the degree of doctor of philosophy, and hereby certify that, in their opinion, it is worthy of acceptance. _____________________________ Dr. J. Chris Pires _____________________________ Dr. James A. Birchler _____________________________ Dr. Paula McSteen _____________________________ Dr. Gavin Conant ACKNOWLEDGEMENTS I would first like to thank my beautiful wife and sweetheart Melinda for her constant companionship, support, and sacrifice over the past five years. Also my three children: Nathan, Sam, and Emma. The four of you have been, and continue to be my inspiration, and my happiness. I also want to thank my parents, Shelley and Kevin Washburn, who instilled in me a love for learning and for hard work. This degree is for you as well. I also thank my advisor Chris for being the most supportive, helpful, and forward- thinking mentor I have ever had the privilege of associating with. I credit you with the success I have had in grant writing during my Ph.D., and with many of the life skills I have learned. My co-advisor Jim has also been an incredible help. -

Molecular Biogeography of Grasses and Tropical Grasslands Jan Hackel
Molecular biogeography of grasses and tropical grasslands Jan Hackel To cite this version: Jan Hackel. Molecular biogeography of grasses and tropical grasslands. Vegetal Biology. Université Paul Sabatier - Toulouse III, 2017. English. NNT : 2017TOU30222. tel-03123970 HAL Id: tel-03123970 https://tel.archives-ouvertes.fr/tel-03123970 Submitted on 28 Jan 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Molecular biogeography of grasses and tropical grasslands Biogéographie moléculaire des graminées et des savanes tropicales Jan Hackel 13 December 2017 Doctoral dissertation Thèse de doctorat Université Toulouse III – Paul Sabatier Laboratoire Evolution et Diversité Biologique Supervisor/Directeur de thèse: Guillaume Besnard Examination board/Jury de thèse: Monique Gardes, Université Toulouse III – Paul Sabatier Alex Baumel, Aix-Marseille Université (rapporteur) Peter Linder, Universität Zürich Yves Vigouroux, IRD Montpellier (rapporteur) Acknowledgements I would like to thank a number of people for accompanying me through these last three years. First of all, Guillaume Besnard was a great supervisor, always available for feedback, with this intuition for the curious details, and we spent hours in the afternoon heat of Madagascar sterilising grass leaves. Maria Vorontsova was involved in all parts of this dissertation. -

Border Rivers-Gwydir, New South Wales
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Macro-Climatic Distribution Limits Show Both Niche Expansion and Niche Specialization
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CONICET Digital RESEARCH ARTICLE Macro-Climatic Distribution Limits Show Both Niche Expansion and Niche Specialization among C4Panicoids Lone Aagesen1*, Fernando Biganzoli2, Julia Bena1, Ana C. Godoy-Bürki1, Renata Reinheimer3, Fernando O. Zuloaga1 1 Instituto de Botánica Darwinion (CONICET-ANCEFN), Labarden 200, San Isidro, B1642HYD, Buenos Aires, Argentina, 2 Departamento de Métodos Cuantitativos y Sistemas de Información, Facultad de Agronomía, UBA, Buenos Aires, Argentina, 3 Instituto de Agrobiotecnologia del Litoral, Santa Fe de la Vera Cruz, Santa Fe, Argentina * [email protected] Abstract Grasses are ancestrally tropical understory species whose current dominance in warm OPEN ACCESS open habitats is linked to the evolution of C4 photosynthesis. C4 grasses maintain high rates Citation: Aagesen L, Biganzoli F, Bena J, Godoy- of photosynthesis in warm and water stressed environments, and the syndrome is consid- Bürki AC, Reinheimer R, Zuloaga FO (2016) Macro- ered to induce niche shifts into these habitats while adaptation to cold ones may be compro- Climatic Distribution Limits Show Both Niche Expansion and Niche Specialization among mised. Global biogeographic analyses of C4 grasses have, however, concentrated on C4Panicoids. PLoS ONE 11(3): e0151075. diversity patterns, while paying little attention to distributional limits. Using phylogenetic con- doi:10.1371/journal.pone.0151075 trast analyses, we compared macro-climatic distribution limits among ~1300 grasses from Editor: Frank Alexander Feltus, Clemson University, the subfamily Panicoideae, which includes 4/5 of the known photosynthetic transitions in UNITED STATES grasses. We explored whether evolution of C4 photosynthesis correlates with niche expan- Received: December 16, 2015 sions, niche changes, or stasis at subfamily level and within the two tribes Paniceae and Accepted: February 23, 2016 Paspaleae. -
Part 10 ESP Intro
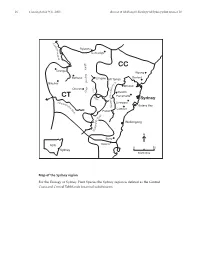
16 Cunninghamia 9(1): 2005 Benson & McDougall, Ecology of Sydney plant species 10 M a c q u Rylstone a r i e Coricudgy R i v e r e g n CC a Orange R Wyong g n i Gosford Bathurst d i Lithgow v Mt Tomah i Blayney D R. y r Windsor C t u a o b Oberon s e x r e s G k Penrith w a R Parramatta CT H i ve – Sydney r n a Abe e Liverpool rcro p m e b Botany Bay ie N R Camden iv Picton er er iv R y l l i Wollongong d n o l l o W N Berry NSW Nowra 050 Sydney kilometres Map of the Sydney region For the Ecology of Sydney Plant Species the Sydney region is defined as the Central Coast and Central Tablelands botanical subdivisions. Cunninghamia 9(1): 2005 Benson & McDougall, Ecology of Sydney plant species 10 17 Ecology of Sydney plant species Part 10 Monocotyledon families Lemnaceae to Zosteraceae Doug Benson and Lyn McDougall Royal Botanic Gardens and Domain Trust, Sydney, AUSTRALIA 2000. Email: [email protected] Abstract: Ecological data in tabular form are provided on 668 plant species of the families Lemnaceae to Zosteraceae, 505 native and 163 exotics, occurring in the Sydney region, defined by the Central Coast and Central Tablelands botanical subdivisions of New South Wales (approximately bounded by Lake Macquarie, Orange, Crookwell and Nowra). Relevant Local Government Areas are Auburn, Ashfield, Bankstown, Bathurst, Baulkham Hills, Blacktown, Blayney, Blue Mountains, Botany, Burwood, Cabonne, Camden, Campbelltown, Canada Bay, Canterbury, Cessnock, Crookwell, Evans, Fairfield, Greater Lithgow, Gosford, Hawkesbury, Holroyd, Hornsby, Hunters Hill, Hurstville, Kiama, Kogarah, Ku-ring-gai, Lake Macquarie, Lane Cove, Leichhardt, Liverpool, Manly, Marrickville, Mosman, Mulwaree, North Sydney, Oberon, Orange, Parramatta, Penrith, Pittwater, Randwick, Rockdale, Ryde, Rylstone, Shellharbour, Shoalhaven, Singleton, South Sydney, Strathfield, Sutherland, Sydney City, Warringah, Waverley, Willoughby, Wingecarribee, Wollondilly, Wollongong, Woollahra and Wyong. -

Torres Strait, Queensland
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

The Impact of Multiple Molecular and Morphological Data Sets on the Phylogenetic Reconstruction of Subtribe Neurachninae (Poaceae: Panicoideae: Paniceae)
Australian Systematic Botany, 2021, 34, 227–251 © CSIRO 2021 https://doi.org/10.1071/SB20015_AC Supplementary material The impact of multiple molecular and morphological data sets on the phylogenetic reconstruction of subtribe Neurachninae (Poaceae: Panicoideae: Paniceae) E. J. ThompsonA,B and Melodina FabilloA AQueensland Herbarium, Department of Environment and Science, Brisbane Botanic Gardens, Mt Coot-tha Road, Toowong, Qld 4066, Australia. BCorresponding author. Email: [email protected]; [email protected] Page 1 of 5 Table S1. Primers used in the amplification of selected DNA regions in Neurachninae, its allies and outgroup species (Panicoideae) Locus Amplification primers (5′ to 3′) PCR conditions Reference ITS ITS1: TTG CGT TCA AAG ACT CGA TGA 94°C, 4 min; 30× (94°C, 1:30 min; 52°C, 0:30 min; 72°C, Grass Phylogeny Working Group II (2012); Teerawatananon et al. (2011) ITS2: AAC AAC TCT CAG CAA CGG 1:00 min) 72°C, 10 min matK matK1412SR: CTG ATA CAT AAG AGT TRT AT 94°C, 3 min; 30× (94°C, 1:00 min; 49°C, 0:30 min; 72°C, Christin et al. (2012); Soreng et al. (2015); Zimmermann et al. (2013) trnK391F: ATC TGG GTT GCT AAC TCA ATG G 1:00 min) 72°C, 10 min ndhF ndhF1311F: ACT GCA GGA TTA ACT GCG TT 94°C, 4 min; 30× (94°C, 1:30 min; 48°C, 0:30 min; 72°C, Christin et al. (2012); Grass Phylogeny Working Group II (2012); Morrone et al. (2012); ndhF2091R: GAC CCA CTC CAT TGG TAA TTC 1:00 min) 72°C, 10 min Soreng et al. -

Appendix 1 – Supporting Tables
Appendix 1 – Supporting tables Table S 1.1 Sampled species, their taxonomic affinities (clade) and GenBank accession numbers for the three chloroplast regions matK, ndhF and rbcL. Clade name abbreviations: _BAM = subfam. Bambusoideae; _ORY = subfam. Oryzoideae; _PAN = subfam. Panicoideae; AVE = Aveneae; BRA = Brachypodieae; BRY = Brachyelytreae; DIA = Diarrheneae; DUT = Duthieae; LYG = Lygeeae; MEL = Meliceae; NAR = Nardeae; POE = Poeae; STI = Stipeae; TRI = Triticeae. Clade Species matK ndhF rbcL _BAM Arundinaria tecta KC817463 KC817463 KC817463 _BAM Bambusa bambos KJ870988 KJ870988 KJ870988 _BAM Cryptochloa strictiflora JX235348 JX235348 JX235348 _ORY Ehrharta erecta AY792568 AM887887 LN907926 _ORY Oryza sativa JN861110 JN861110 JN861110 _PAN Reynaudia filiformis HE574132 JN604704 HE573433 _PAN Sorghum bicolor NC_008602 NC_008602 NC_008602 _PAN Zea mays X86563 X86563 X86563 AVE Agrostis canina FJ231115 MH888123* JN893786 AVE Agrostis capillaris FJ231112 JX438119 AY395527 AVE Agrostis stolonifera EF115543 EF115543 EF115543 AVE Agrostis tenerrima DQ786877 DQ786805 n/a AVE Agrostis vinealis FJ231113 n/a JN893785 AVE Ammophila arenaria AM234561 JX438118 JN890705 AVE Ammophila breviligulata KM974730 KM974730 KM974730 AVE Amphibromus scabrivalvis DQ786882 DQ786810 n/a AVE Anthoxanthum arcticum KC474036 n/a JN965581 AVE Anthoxanthum monticola KC474042 n/a JN965577 AVE Anthoxanthum nitens KM974740 KM974740 KM974740 AVE Anthoxanthum nivale LN906649 LN908047 LN907884 AVE Anthoxanthum odoratum KM974732 KM974732 KM974732 AVE Arrhenatherum elatius