Comparative Value of Fish Meal Alternatives As Protein Sources In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fisheries Centre
Fisheries Centre The University of British Columbia Working Paper Series Working Paper #2015 - 80 Reconstruction of Syria’s fisheries catches from 1950-2010: Signs of overexploitation Aylin Ulman, Adib Saad, Kyrstn Zylich, Daniel Pauly and Dirk Zeller Year: 2015 Email: [email protected] This working paper is made available by the Fisheries Centre, University of British Columbia, Vancouver, BC, V6T 1Z4, Canada. Reconstruction of Syria’s fisheries catches from 1950-2010: Signs of overexploitation Aylin Ulmana, Adib Saadb, Kyrstn Zylicha, Daniel Paulya, Dirk Zellera a Sea Around Us, Fisheries Centre, University of British Columbia, 2202 Main Mall, Vancouver, BC, V6T 1Z4, Canada b President of Syrian National Committee for Oceanography, Tishreen University, Faculty of Agriculture, P.O. BOX; 1408, Lattakia, Syria [email protected] (corresponding author); [email protected]; [email protected]; [email protected]; [email protected] ABSTRACT Syria’s total marine fisheries catches were estimated for the 1950-2010 time period using a reconstruction approach which accounted for all fisheries removals, including unreported commercial landings, discards, and recreational and subsistence catches. All unreported estimates were added to the official data, as reported by the Syrian Arab Republic to the United Nation’s Food and Agriculture Organization (FAO). Total reconstructed catch for 1950-2010 was around 170,000 t, which is 78% more than the amount reported by Syria to the FAO as their national catch. The unreported components added over 74,000 t of unreported catches, of which 38,600 t were artisanal landings, 16,000 t industrial landings, over 4,000 t recreational catches, 3,000 t subsistence catches and around 12,000 t were discards. -

Download E-Book (PDF)
African Journal of Biotechnology Volume 14 Number 33, 19 August, 2015 ISSN 1684-5315 ABOUT AJB The African Journal of Biotechnology (AJB) (ISSN 1684-5315) is published weekly (one volume per year) by Academic Journals. African Journal of Biotechnology (AJB), a new broad-based journal, is an open access journal that was founded on two key tenets: To publish the most exciting research in all areas of applied biochemistry, industrial microbiology, molecular biology, genomics and proteomics, food and agricultural technologies, and metabolic engineering. Secondly, to provide the most rapid turn-around time possible for reviewing and publishing, and to disseminate the articles freely for teaching and reference purposes. All articles published in AJB are peer- reviewed. Submission of Manuscript Please read the Instructions for Authors before submitting your manuscript. The manuscript files should be given the last name of the first author Click here to Submit manuscripts online If you have any difficulty using the online submission system, kindly submit via this email [email protected]. With questions or concerns, please contact the Editorial Office at [email protected]. Editor-In-Chief Associate Editors George Nkem Ude, Ph.D Prof. Dr. AE Aboulata Plant Breeder & Molecular Biologist Plant Path. Res. Inst., ARC, POBox 12619, Giza, Egypt Department of Natural Sciences 30 D, El-Karama St., Alf Maskan, P.O. Box 1567, Crawford Building, Rm 003A Ain Shams, Cairo, Bowie State University Egypt 14000 Jericho Park Road Bowie, MD 20715, USA Dr. S.K Das Department of Applied Chemistry and Biotechnology, University of Fukui, Japan Editor Prof. Okoh, A. I. N. -

Fish Species of Saskatchewan
Introduction From the shallow, nutrient -rich potholes of the prairies to the clear, cool rock -lined waters of our province’s north, Saskatchewan can boast over 50,000 fish-bearing bodies of water. Indeed, water accounts for about one-eighth, or 80,000 square kilometers, of this province’s total surface area. As numerous and varied as these waterbodies are, so too are the types of fish that inhabit them. In total, Saskatchewan is home to 67 different fish species from 16 separate taxonomic families. Of these 67, 58 are native to Saskatchewan while the remaining nine represent species that have either been introduced to our waters or have naturally extended their range into the province. Approximately one-third of the fish species found within Saskatchewan can be classed as sportfish. These are the fish commonly sought out by anglers and are the best known. The remaining two-thirds can be grouped as minnow or rough-fish species. The focus of this booklet is primarily on the sportfish of Saskatchewan, but it also includes information about several rough-fish species as well. Descriptions provide information regarding the appearance of particular fish as well as habitat preferences and spawning and feeding behaviours. The individual species range maps are subject to change due to natural range extensions and recessions or because of changes in fisheries management. "...I shall stay him no longer than to wish him a rainy evening to read this following Discourse; and that, if he be an honest Angler, the east wind may never blow when he goes a -fishing." The Compleat Angler Izaak Walton, 1593-1683 This booklet was originally published by the Saskatchewan Watershed Authority with funds generated from the sale of angling licences and made available through the FISH AND WILDLIFE DEVELOPMENT FUND. -

Northern Pike Esox Lucius ILLINOIS RANGE
northern pike Esox lucius Kingdom: Animalia FEATURES Phylum: Chordata The northern pike's average life span is eight to 10 Class: Osteichthyes years. The average weight is two pounds. It may Order: Esociformes attain a maximum length of 53 inches. The fins are rounded, and all except the pectoral fins have dark Family: Esocidae spots. Scales are present on the cheek and half of ILLINOIS STATUS the gill cover. The eyes are yellow. The long, green body has yellow spots on the sides. The belly is common, native white to dark yellow. The duckbill-shaped snout is easily seen. BEHAVIORS The northern pike lives in lakes, rivers and marshes. It prefers water without strong currents and with many plants. This fish reaches maturity at age two to three years. Spawning occurs in March. The female deposits up to 150,000 eggs that are scattered in marshy areas or other shallow water areas. Eggs hatch in 12-14 days. This fish eats fishes, insects, crayfish, frogs and reptiles. ILLINOIS RANGE © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. close up of head close up of side © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. © Engbretson Underwater Photography adult Aquatic Habitats lakes, ponds and reservoirs; rivers and streams; marshes Woodland Habitats none Prairie and Edge Habitats none © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources.. -

Fisheries Full Issue PDF Volume 39, Issue 3
This article was downloaded by: [American Fisheries Society] On: 24 March 2014, At: 13:30 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Fisheries Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/ufsh20 Full Issue PDF Volume 39, Issue 3 Published online: 21 Mar 2014. To cite this article: (2014) Full Issue PDF Volume 39, Issue 3, Fisheries, 39:3, 97-144, DOI: 10.1080/03632415.2014.902705 To link to this article: http://dx.doi.org/10.1080/03632415.2014.902705 PLEASE SCROLL DOWN FOR ARTICLE Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and should be independently verified with primary sources of information. Taylor and Francis shall not be liable for any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoever or howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use of the Content. This article may be used for research, teaching, and private study purposes. -

APPENDIX M Common and Scientific Species Names
Bay du Nord Development Project Environmental Impact Statement APPENDIX M Common and Scientific Species Names Bay du Nord Development Project Environmental Impact Statement Common and Species Names Common Name Scientific Name Fish Abyssal Skate Bathyraja abyssicola Acadian Redfish Sebastes fasciatus Albacore Tuna Thunnus alalunga Alewife (or Gaspereau) Alosa pseudoharengus Alfonsino Beryx decadactylus American Eel Anguilla rostrata American Plaice Hippoglossoides platessoides American Shad Alosa sapidissima Anchovy Engraulidae (F) Arctic Char (or Charr) Salvelinus alpinus Arctic Cod Boreogadus saida Atlantic Bluefin Tuna Thunnus thynnus Atlantic Cod Gadus morhua Atlantic Halibut Hippoglossus hippoglossus Atlantic Mackerel Scomber scombrus Atlantic Salmon (landlocked: Ouananiche) Salmo salar Atlantic Saury Scomberesox saurus Atlantic Silverside Menidia menidia Atlantic Sturgeon Acipenser oxyrhynchus oxyrhynchus Atlantic Wreckfish Polyprion americanus Barndoor Skate Dipturus laevis Basking Shark Cetorhinus maximus Bigeye Tuna Thunnus obesus Black Dogfish Centroscyllium fabricii Blue Hake Antimora rostrata Blue Marlin Makaira nigricans Blue Runner Caranx crysos Blue Shark Prionace glauca Blueback Herring Alosa aestivalis Boa Dragonfish Stomias boa ferox Brook Trout Salvelinus fontinalis Brown Bullhead Catfish Ameiurus nebulosus Burbot Lota lota Capelin Mallotus villosus Cardinal Fish Apogonidae (F) Chain Pickerel Esox niger Common Grenadier Nezumia bairdii Common Lumpfish Cyclopterus lumpus Common Thresher Shark Alopias vulpinus Crucian Carp -

Esox Lucius) Ecological Risk Screening Summary
Northern Pike (Esox lucius) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, February 2019 Web Version, 8/26/2019 Photo: Ryan Hagerty/USFWS. Public Domain – Government Work. Available: https://digitalmedia.fws.gov/digital/collection/natdiglib/id/26990/rec/22. (February 1, 2019). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2019a): “Circumpolar in fresh water. North America: Atlantic, Arctic, Pacific, Great Lakes, and Mississippi River basins from Labrador to Alaska and south to Pennsylvania and Nebraska, USA [Page and Burr 2011]. Eurasia: Caspian, Black, Baltic, White, Barents, Arctic, North and Aral Seas and Atlantic basins, southwest to Adour drainage; Mediterranean basin in Rhône drainage and northern Italy. Widely distributed in central Asia and Siberia easward [sic] to Anadyr drainage (Bering Sea basin). Historically absent from Iberian Peninsula, Mediterranean France, central Italy, southern and western Greece, eastern Adriatic basin, Iceland, western Norway and northern Scotland.” Froese and Pauly (2019a) list Esox lucius as native in Armenia, Azerbaijan, China, Georgia, Iran, Kazakhstan, Mongolia, Turkey, Turkmenistan, Uzbekistan, Albania, Austria, Belgium, Bosnia Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Macedonia, Moldova, Monaco, 1 Netherlands, Norway, Poland, Romania, Russia, Serbia, Slovakia, Slovenia, Sweden, Switzerland, United Kingdom, Ukraine, Canada, and the United States (including Alaska). From Froese and Pauly (2019a): “Occurs in Erqishi river and Ulungur lake [in China].” “Known from the Selenge drainage [in Mongolia] [Kottelat 2006].” “[In Turkey:] Known from the European Black Sea watersheds, Anatolian Black Sea watersheds, Central and Western Anatolian lake watersheds, and Gulf watersheds (Firat Nehri, Dicle Nehri). -
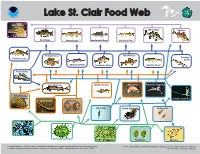
Lake St. Clair Food Web MENT of C
ATMOSPH ND ER A I C C I A N D A M E I C N O I S L T A R N A T O I I O T N A N U E .S C .D R E E PA M RT OM Lake St. Clair Food Web MENT OF C Sea Lamprey White Bass Walleye Rock Bass Muskellunge Smallmouth Bass Northern Pike Pumpkinseed Yellow Perch Rainbow Common Carp Smelt Channel Catfish Freshwater Drum Spottail Shiner Round Goby Lake Sturgeon Mayfly nymphs Gammarus Raptorial waterflea Zebra/Quagga mussels Invasive waterflea Chironomids Mollusks Calanoids Native waterflea Cyclopoids Diatoms Green algae Blue-green algae Flagellates Rotifers Foodweb based on “Impact of exotic invertebrate invaders on food web structure and function in the Great Lakes: NOAA, Great Lakes Environmental Research Laboratory, 4840 S. State Road, Ann Arbor, MI A network analysis approach” by Mason, Krause, and Ulanowicz, 2002 - Modifications for Lake St. Clair, 2009. 734-741-2235 - www.glerl.noaa.gov Lake St. Clair Food Web Sea Lamprey Planktivores/Benthivores Sea lamprey (Petromyzon marinus). An aggressive, non-native parasite that Lake Sturgeon (Acipenser fulvscens). Endangered over most of its historic fastens onto its prey and rasps out a hole with its rough tongue. range. Its diet commonly includes small clams, snails, crayfish, sideswimmers, aquatic insect larvae, algae, and other plant matter. Piscivores (Fish Eaters) Macroinvertebrates White bass (Morone Chrysops). Prefers clear open water in lakes and large rivers. Chironomids/Oligochaetes. Larval insects and worms that live on the lake Visual feeders, uses sight instead of smell to find prey. -

Current Ocean Wise Approved Canadian MSC Fisheries
Current Ocean Wise approved Canadian MSC Fisheries Updated: November 14, 2017 Legend: Blue - Ocean Wise Red - Not Ocean Wise White - Only specific areas or gear types are Ocean Wise Species Common Name Latin Name MSC Fishery Name Gear Location Reason for Exception Clam Clearwater Seafoods Banquereau and Banquereau Bank Artic surf clam Mactromeris polynyma Grand Banks Arctic surf clam Hydraulic dredges Grand Banks Crab Snow Crab Chionoecetes opilio Gulf of St Lawrence snow crab trap Conical or rectangular crab pots (traps) North West Atlantic - Nova Scotia Snow Crab Chionoecetes opilio Scotian shelf snow crab trap Conical or rectangular crab pots (traps) North West Atlantic - Nova Scotia Snow Crab Chionoecetes opilio Newfoundland & Labrador snow crab Pots Newfoundland & Labrador Flounder/Sole Yellowtail flounder Limanda ferruginea OCI Grand Bank yellowtail flounder trawl Demersal trawl Grand Banks Haddock Trawl Bottom longline Gillnet Hook and Line CAN - Scotian shelf 4X5Y Trawl Bottom longline Gillnet Atlantic haddock Melangrammus aeglefinus Canada Scotia-Fundy haddock Hook and Line CAN - Scotian shelf 5Zjm Hake Washington, Oregon and California North Pacific hake Merluccius productus Pacific hake mid-water trawl Mid-water Trawl British Columbia Halibut Pacific Halibut Hippoglossus stenolepis Canada Pacific halibut (British Columbia) Bottom longline British Columbia Longline Nova Scotia and Newfoundland Gillnet including part of the Grand banks and Trawl Georges bank, NAFO areas 3NOPS, Atlantic Halibut Hippoglossus hippoglossus Canada -

(Muğl ) O Ho Kly Ġġ Ġ Ġcġl
NK NĠ Ġ Ġ N ĠLĠML Ġ N Ġ OK O Ġ G LL K KÖ ĠN (MUĞL ) O HO K L Y Ġġ Ġ ĠCĠLĠĞĠ Y PIL N L K (Dicentrarchus labrax) ÇĠPU (Sparus aurata) LIKL IN ULUN N K OP Ġ L ĠN LĠ L NM Ġ Quyet PHAN VAN U NL Ġ N ĠLĠM LI ANKARA 2020 Her hakkı saklıdır Ö DOKTORA TEZI G LL K KOR Z N (MUĞLA) O SHOR KA SL R Y T ŞT R C L Ğ YAPILAN L VR K (Dicentrarchus labrax) V ÇIPURA (Sparus aurata) ALIKLARIN A ULUNAN KTOPARAZ TL R N L RL NM S Quyet PHAN VAN Ankara niversitesi Fen ilimleri nstit s Su r nleri Ana ilim alı anı man: Prof r Hijran YAVUZCAN u tez alı masında, G ll k Körfezinde ulunan off-shore kafeslerde yeti tiricili i yapılan levrek (Dicentrarchus labrax) ve ipura (Sparus aurata) alıklarında ulunan ektoparazitler mevsimsel olarak incelenmi tir Çalı mada 175 adet levrek ve 175 ipura olmak zere toplam 35 alık incelenmi tir alıklarda yapılan incelemeler soncunda genel parazit prevalansı levrek alıklarında %97 14, ipura alıklarında ise %5 8 olarak elirlenmi tir Levrek alıklarında tespit edilen ektoparazitlerden Diplectanum aequans, Lernanthropus kroyeri ve Amyloodinium ocellatum t rlerinin prevalansı sırasıyla %82.28, %58 85 ve %4 57 olarak elirlenmi tir Levrek alıklarında parazitlere ait ortalama bolluk de erleri; D. aequans i in 4 61± 56, L. kroyeri i in 1 33± 15 ve A. ocellatum i in 0.10± 4 olarak saptanmı tır Levrek alıklarında parazitlerin ortalama yo unlukları, D. aequans i in 5 61± 56, L. -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -

Victor Buchet
Master‘s thesis Impact assessment of invasive flora species in Posidonia oceanica meadows on fish assemblage: an influence on local fisheries? The case study of Lipsi Island, Greece. Victor Buchet Advisor: Michael Honeth University of Akureyri Faculty of Business and Science University Centre of the Westfjords Master of Resource Management: Coastal and Marine Management Ísafjörður, September 2014 Supervisory Committee Advisor: - Micheal Honeth, Tobacco Caye Marine Station, Belize. Reader: - Zoi I. Konstantinou, MSc. - Ph.D. Program Director: - Dagný Arnarsdóttir, MSc. Victor Buchet Impact assessment of invasive flora species in Posidonia oceanica meadows on fish assemblage: an influence on local fisheries? The case study of Lipsi Island, Greece. 45 ECTS thesis submitted in partial fulfilment of a Master of Resource Management degree in Coastal and Marine Management at the University Centre of the Westfjords, Suðurgata 12, 400 Ísafjörður, Iceland Degree accredited by the University of Akureyri, Faculty of Business and Science, Borgir, 600 Akureyri, Iceland Copyright © 2014 Buchet All rights reserved Printing: Háskólaprent, Reykjavík, September 2014 ii Declaration I hereby confirm that I am the sole author of this thesis and it is a product of my own academic research. Victor Buchet Student‘s name iii Abstract Seagrasses are one of the most valuable coastal ecosystems with regards to biodiversity and ecological services, whose diminishing presence plays a significant role in the availability of resources for local communities and human well-being. At the same time, Invasive Alien Species (IAS) are considered as one of the biggest threats to marine worldwide biodiversity. In the Mediterranean, the issue of IAS is one which merits immediate attention; where habitat alteration caused by the human-mediated arrival of new species is a common concern.